Mastering Denaturing Protein Gel Electrophoresis: A Complete Guide to Sample Preparation for Reproducible Results
This article provides a comprehensive guide for researchers, scientists, and drug development professionals on preparing high-quality samples for denaturing protein gel electrophoresis (SDS-PAGE).
Mastering Denaturing Protein Gel Electrophoresis: A Complete Guide to Sample Preparation for Reproducible Results
Abstract
This article provides a comprehensive guide for researchers, scientists, and drug development professionals on preparing high-quality samples for denaturing protein gel electrophoresis (SDS-PAGE). It covers the foundational principles of SDS-PAGE, detailed step-by-step methodological protocols for various sample types, systematic troubleshooting for common artifacts, and validation techniques to ensure data accuracy and reproducibility. By integrating best practices for lysis, denaturation, reduction, and quantification, this guide aims to enhance experimental reliability in proteomic research and biomarker discovery.
The Principles of Denaturing Protein Electrophoresis: Why Sample Prep is Everything
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) is a foundational technique in molecular biology and biochemistry that enables the separation of proteins based solely on their molecular mass [1]. Developed by Ulrich K. Laemmli in 1970, this method has become one of the most widely cited techniques in scientific literature, with over 300,000 citations to date [2] [3]. The fundamental power of SDS-PAGE lies in its ability to negate the influence of a protein's inherent charge and three-dimensional structure, allowing researchers to separate complex protein mixtures with high resolution and reproducibility [1] [4].
The technique remains indispensable across diverse scientific fields, from basic academic research to biopharmaceutical development [5] [6]. In drug development, SDS-PAGE serves critical quality control functions, enabling the characterization of therapeutic proteins, including monoclonal antibodies, vaccines, and viral vectors [2]. The method continues to evolve with advancements in automation, precast gel systems, and digital imaging technologies, yet its core separation principles remain unchanged [7].
Core Principles of Separation
The Role of SDS in Protein Denaturation and Uniform Charge Conferment
SDS (Sodium Dodecyl Sulfate) is an anionic detergent that performs two essential functions in protein separation. First, it acts as a powerful denaturant, disrupting the non-covalent interactions that maintain secondary and tertiary protein structures [8]. The SDS molecule contains both a hydrophobic hydrocarbon chain and a hydrophilic sulfate group, allowing it to interact with and unfold both polar and nonpolar regions of proteins [1] [3].
Second, SDS binds to the denatured polypeptide chains with high affinity—approximately 1.4 grams of SDS per gram of protein, corresponding to one SDS molecule per two amino acids [3]. This uniform coating imparts a strong negative charge to all proteins in the mixture, effectively masking their intrinsic charges [1] [4]. The result is that all proteins gain a similar charge-to-mass ratio, eliminating charge and shape as factors in their electrophoretic mobility [8]. Consequently, separation occurs based primarily on molecular size rather than native charge or structural characteristics [4].
Polyacrylamide Gel as a Molecular Sieve
The polyacrylamide gel matrix serves as a molecular sieve that differentially retards the movement of proteins based on their size [1]. The gel forms through the polymerization of acrylamide monomers cross-linked by bis-acrylamide, creating a three-dimensional network with controllable pore sizes [3]. The polymerization reaction is catalyzed by ammonium persulfate (APS) and tetramethylethylenediamine (TEMED), which generate free radicals to initiate the process [1] [8].
The porosity of the gel is determined by the concentration of acrylamide, with higher percentages creating smaller pores that provide better resolution for lower molecular weight proteins [8]. This relationship allows researchers to select gel compositions optimized for their protein size range of interest (Table 1) [1].
Table 1: Recommended Acrylamide Concentrations for Separating Proteins of Different Molecular Mass Ranges
| Acrylamide Percentage (%) | Effective Separation Range (kDa) |
|---|---|
| 8% | 30-200 |
| 10% | 20-150 |
| 12% | 10-100 |
| 15% | 5-80 |
The Discontinuous Buffer System
SDS-PAGE employs a discontinuous buffer system that enhances separation resolution through both pH and gel porosity differences [8]. The system comprises two distinct gel layers: a stacking gel (pH ~6.8) with lower acrylamide concentration (~4%) positioned above a resolving gel (pH ~8.8) with higher acrylamide concentration (typically 8-15%) [1] [3].
The key to this system lies in the differential mobility of ions within the electrical field. In the stacking gel, glycine from the running buffer exists primarily as zwitterions with minimal net charge, migrating slowly compared to chloride ions (leading ions) and protein-SDS complexes (trailing ions) [8]. This creates a steep voltage gradient that concentrates proteins into a narrow zone before they enter the resolving gel [1]. Upon reaching the resolving gel at higher pH, glycine molecules become deprotonated, gaining negative charge and migrating faster, depositing the protein stack at the top of the resolving gel where actual separation by size occurs [8].
Materials and Reagents
Table 2: Essential Reagents for SDS-PAGE and Their Functions
| Reagent | Function | Key Specifications |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) | Denatures proteins and confers uniform negative charge [1] [4] | Typically 1-2% in sample buffer; critical micelle concentration of 7-10 mM [3] |
| Acrylamide/Bis-acrylamide | Forms the porous gel matrix for molecular sieving [1] | Ratio of bis-acrylamide:acrylamide typically ~1:35; concentration varies from 4-20% depending on protein size [1] |
| APS (Ammonium Persulfate) and TEMED | Catalyzes acrylamide polymerization [1] [8] | TEMED stabilizes persulfate free radicals generated by APS to initiate polymerization [1] |
| Tris-HCl Buffers | Maintains pH during electrophoresis [8] | Stacking gel: pH 6.8; Resolving gel: pH 8.8; pKa of Tris (8.1) ideal for biological systems [3] [8] |
| Glycine | Key ion in discontinuous buffer system for stacking [8] | Running buffer pH 8.3; charge state changes with pH enable stacking effect [8] |
| Reducing Agents (DTT, BME, TCEP) | Breaks disulfide bonds for complete denaturation [1] [9] | DTT (10-100 mM), BME (2.5%), or TCEP (50 mM); added fresh before heating [9] [3] |
| Tracking Dye (Bromophenol Blue) | Visualizes migration progress during electrophoresis [8] | Small anionic dye migrates ahead of proteins; indicates buffer front [3] |
Detailed Experimental Protocol
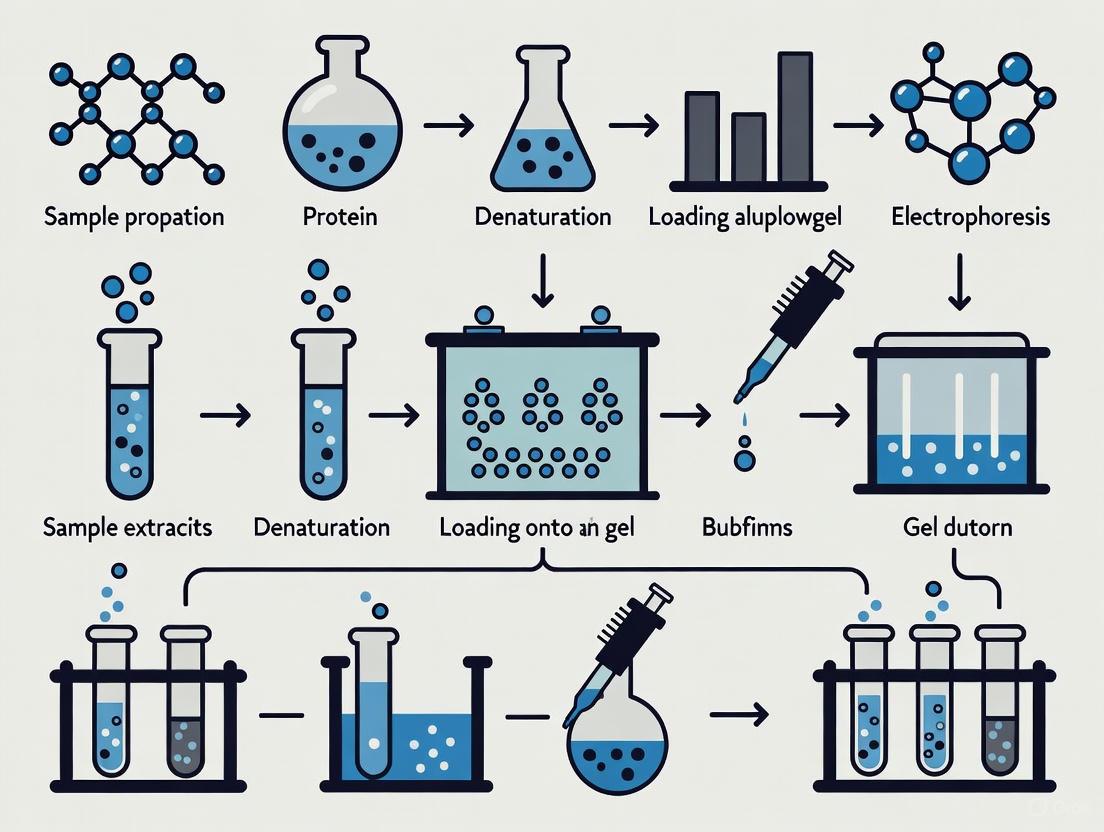
Sample Preparation Protocol
Proper sample preparation is critical for successful SDS-PAGE separation and requires careful attention to denaturation and reduction conditions:
Sample Buffer Preparation: Prepare 2X Laemmli sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, and 0.01% bromophenol blue [8]. For reduced conditions, add fresh reducing agent immediately before use: 50 mM DTT, 2.5% β-mercaptoethanol, or 50 mM TCEP [9].
Protein Denaturation: Mix protein sample with equal volume of 2X sample buffer. Heat the mixture at 85°C for 2-5 minutes for optimal denaturation [9]. Avoid exceeding 95°C as this can promote protein proteolysis [9]. For proteins with SDS-resistant complexes, heating at 95°C for 5 minutes may be necessary [3].
Special Considerations:
- For cell lysates containing genomic DNA, shear DNA by brief sonication or passage through a small-gauge needle to reduce viscosity [9].
- For samples in high-salt buffers (>100 mM) or containing guanidine-HCl, perform dialysis or protein precipitation/resuspension to avoid gel artifacts [9].
- Centrifuge heated samples at 16,000 × g for 5 minutes to pellet insoluble material before loading [9].
Gel Preparation and Electrophoresis Protocol
The gel preparation process requires precision in both chemistry and timing:
Gel Casting:
- Resolving Gel: Combine appropriate acrylamide percentage for target protein size (Table 1), 375 mM Tris-HCl (pH 8.8), 0.1% SDS, APS, and TEMED [1] [3]. Pour between glass plates and overlay with water-saturated isopropanol or butanol to exclude oxygen and create a flat interface [1] [3]. Allow complete polymerization (typically 15-30 minutes).
- Stacking Gel: After removing overlay, add stacking gel solution (4% acrylamide, 125 mM Tris-HCl pH 6.8, 0.1% SDS, APS, TEMED) and insert sample comb [1]. Polymerize for 15-30 minutes.
Electrophoresis Setup:
- Assemble gel cassette in electrophoresis chamber filled with running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) [8].
- Load samples (typically 10-40 μL) and molecular weight markers into wells.
- Apply constant voltage: 80-100 V through stacking gel, 120-150 V through resolving gel [3]. Run until bromophenol blue front reaches bottom of gel (typically 1-1.5 hours).
Factors Affecting Resolution and Accuracy
Several critical factors influence the resolution and accuracy of SDS-PAGE separation:
Gel Composition Factors: Acrylamide concentration directly determines pore size and resolution range [1] [5]. The degree of cross-linking (bis-acrylamide ratio) affects gel porosity and mechanical stability [5]. Inconsistent polymerization due to improper TEMED/APS ratios or oxygen contamination causes poor resolution [5].
Sample Preparation Factors: Incomplete reduction of disulfide bonds leads to aberrant migration [9]. Overheating during denaturation can cause protein degradation or modification [9]. The protein-to-SDS ratio must be sufficient for complete coating (1.4g SDS:1g protein) [3]. High salt concentrations distort band morphology and migration [9].
Electrophoresis Conditions: Excessive voltage generates heat, causing band smiling and diffusion [5]. Buffer ion depletion from extended runs reduces resolution [5]. Inconsistent buffer pH alters glycine charge states, compromising stacking [8].
Protein-Specific Considerations: Highly hydrophobic proteins may bind excess SDS and migrate anomalously [8]. Glycosylated or phosphorylated proteins may bind less SDS due to modified charge distribution [8]. Proteins with unusual amino acid compositions may exhibit non-standard SDS binding [3].
Advanced Applications and Technological Evolution
While traditional SDS-PAGE remains widely used, new technologies are enhancing protein separation capabilities. Capillary electrophoresis SDS (CE-SDS) systems offer automated, quantitative analysis with minimal manual steps [2]. These systems provide superior reproducibility through automated separation, removing gel-to-gel variability and subjective band intensity assessments [2]. The Maurice CE-SDS system, for example, enables analysis of various biotherapeutic molecules including monoclonal antibodies, bispecific antibodies, ADCs, and viral vectors [2].
The field continues to evolve with innovations in microfluidic platforms, digital imaging, and artificial intelligence-driven band recognition algorithms [6] [7]. These advancements are particularly valuable in biopharmaceutical applications where precise characterization of protein therapeutics is essential for regulatory compliance [2]. Despite these technological advances, the fundamental principles of SDS-mediated protein separation remain central to modern protein analysis.
In the realm of protein research, sample preparation is a critical step that fundamentally determines the success of downstream analysis. For denaturing protein gel electrophoresis, proper sample treatment ensures accurate separation, identification, and characterization of protein components. Central to this process is sodium dodecyl sulfate (SDS), an anionic detergent that performs two essential functions: it denatures proteins into linear chains and confers upon them a uniform negative charge [10] [11]. This dual action eliminates the influence of native protein structure and intrinsic charge, enabling separation primarily based on molecular weight [12] [3]. Within the context of a broader thesis on sample preparation methodologies, this application note details the mechanistic role of SDS and provides standardized protocols for its application in denaturing gel electrophoresis, serving the needs of researchers, scientists, and drug development professionals who require reproducible and reliable protein analysis.
The Dual Mechanism of SDS Action
SDS operates through two interconnected biochemical mechanisms that transform complex three-dimensional protein structures into uniform linear molecules amenable to electrophoretic separation.
Protein Denaturation and Linearization
SDS effectively disrupts the higher-order structures of proteins, including secondary, tertiary, and quaternary arrangements, with the exception of disulfide bonds which require reducing agents for cleavage [10] [13]. The amphipathic nature of SDS enables this denaturation; its hydrophobic hydrocarbon tail interacts strongly with nonpolar regions of the protein, while its hydrophilic sulfate ionic group remains exposed to the aqueous environment [3] [14]. This interaction dissolves hydrophobic areas and breaks non-covalent ionic bonds within the protein structure [10]. The result is the transformation of precisely folded globular proteins into extended, linear polypeptide chains [11] [14], often described as resembling "overcooked spaghetti" [10]. This linearization is crucial as it standardizes the shape of all proteins, eliminating variations in electrophoretic mobility caused by differences in three-dimensional conformation.
Charge Masking and Uniform Charge-to-Mass Ratio
Following denaturation, SDS binds tenaciously to the protein backbone at an approximately constant ratio of 1.4 grams of SDS per 1 gram of protein [3] [15]. Given that each SDS molecule contributes a strong negative charge, the cumulative effect is a protein complex possessing a significant net negative charge that effectively masks the protein's intrinsic charge derived from its amino acid composition [14] [13]. Since the number of SDS molecules binding to a protein is proportional to the protein's length (number of amino acids) [11], this results in all SDS-coated proteins having a nearly identical charge-to-mass ratio [14] [15]. This charge uniformity ensures that during electrophoresis, proteins migrate strictly according to molecular size rather than their inherent electrical properties [12] [14].
Table 1: Key Properties and Actions of SDS in Protein Denaturation
| Property/Action | Description | Functional Significance |
|---|---|---|
| Chemical Nature | Anionic detergent with hydrophobic tail and hydrophilic sulfate group [10] [13] | Enables interaction with both polar and nonpolar protein regions |
| Binding Ratio | ~1.4 g SDS / 1 g protein [3] [15] | Creates uniform charge-to-mass ratio across different proteins |
| Denaturation Effect | Disrupts hydrogen bonds and hydrophobic interactions [10] | Unfolds proteins into linear chains, eliminating structural variability |
| Critical Micelle Concentration | 7-10 mM in aqueous solutions [3] | Determines effective monomer concentration available for protein binding |
| Charge Contribution | Two negative charges per SDS molecule [11] | Overwhelms intrinsic protein charge, ensuring consistent negative charge |
Comprehensive Reagent System for Denaturing SDS-PAGE
Successful denaturing electrophoresis requires a carefully formulated system of reagents that work in concert with SDS to achieve optimal protein separation.
Table 2: Essential Research Reagents for Denaturing SDS-PAGE
| Reagent | Composition/Type | Primary Function |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) | Anionic detergent (C12H25NaO4S) [13] | Denatures proteins and imparts uniform negative charge [11] [14] |
| Reducing Agent | β-mercaptoethanol, DTT, or DTE [10] [3] | Cleaves disulfide bonds to complete protein unfolding [10] |
| Sample Buffer | Tris-HCl, SDS, glycerol, bromophenol blue, reducing agent [15] [13] | Denatures proteins, provides density for loading, and visual tracking |
| Polyacrylamide Gel | Acrylamide, bis-acrylamide, SDS, Tris buffer, catalysts (APS & TEMED) [3] [13] | Forms sieving matrix for size-based separation [12] [11] |
| Electrophoresis Buffer | Tris, glycine, SDS [14] [13] | Conducts current and maintains pH for electrophoresis |
| Molecular Weight Marker | Pre-stained or unstained proteins of known molecular weights [3] | Provides reference for estimating sample protein sizes |
The denaturing process is typically initiated by incubating protein samples in Laemmli buffer [13], which contains SDS as the primary denaturant, a reducing agent (such as β-mercaptoethanol or dithiothreitol) to break disulfide bonds [10] [3], glycerol to add density for gel loading, and a tracking dye (bromophenol blue) to monitor migration progress [15] [13]. The sample is then heated to 95°C for 5 minutes or 70°C for 10 minutes [3] to facilitate complete denaturation. This heat step further disrupts hydrogen bonds and helps homogenize the sample, particularly important for cell lysates containing DNA [10].
The following diagram illustrates the transformation of native proteins into SDS-bound linear chains and their migration in the electric field:
Quantitative Aspects of SDS-Protein Interactions
The effectiveness of SDS in protein denaturation and charge masking depends on several quantitative parameters that must be carefully controlled for reproducible results.
Table 3: Critical Quantitative Parameters for SDS-Protein Interactions
| Parameter | Optimal Range/Value | Impact on Electrophoresis |
|---|---|---|
| SDS:Protein Ratio | 1.4 g SDS / 1 g protein (constant binding) [3] [15] | Ensures complete charge masking and linearization |
| SDS Concentration in Sample Buffer | 1-2% (w/v) [3] | Maintains denaturing conditions during sample prep |
| SDS Concentration in Running Buffer | 0.1% (standard) or 0.0375% (native SDS-PAGE) [3] [16] | Maintains protein denaturation during electrophoresis |
| Effective Denaturation Concentration | > 0.1 mM (unfolding begins), > 1 mM (most proteins denatured) [3] | Ensures complete protein denaturation |
| Critical Micelle Concentration (CMC) | 7-10 mM (aqueous solutions) [3] | Determines availability of SDS monomers for protein binding |
| Sample Heating Conditions | 95°C for 5 min or 70°C for 10 min [3] | Facilitates complete denaturation and disruption of hydrogen bonds |
It is important to note that while SDS binding is generally uniform across most proteins, certain structural features can lead to anomalous migration. Hydrophobic proteins may bind more SDS than average, while post-translationally modified proteins (e.g., glycosylated or phosphorylated proteins) may bind less SDS due to steric hindrance or altered chemical properties [13]. Additionally, some proteins like tubulin exhibit atypical binding patterns, leading to unexpected migration positions relative to their true molecular weight [15]. These potential anomalies should be considered when interpreting SDS-PAGE results.
Detailed Experimental Protocol for Denaturing SDS-PAGE
Sample Preparation Protocol
Prepare Protein Sample: Mix protein sample with 2X or 4X Laemmli sample buffer to achieve final concentrations of 1-2% SDS, 50-100 mM Tris-HCl (pH 6.8), 5-10% glycerol, 0.001% bromophenol blue, and 1-5% β-mercaptoethanol or 10-100 mM DTT [3] [13].
Denature Proteins: Heat samples at 95°C for 5 minutes or 70°C for 10 minutes using a heat block or water bath [3]. For large proteins (>100 kDa), extend heating time to 10 minutes at 95°C; for small proteins (<20 kDa), reduce heating to 2-3 minutes at 95°C to prevent degradation [10].
Clarify Sample: Centrifuge heated samples at 15,000 rpm for 1 minute at 4°C to pellet any insoluble debris [12]. Use the supernatant for gel loading.
Load Samples: Pipette clarified samples into wells of the SDS-PAGE gel, including appropriate molecular weight markers in one lane [12] [3]. Typical protein loading ranges from 0.1 µg (minimum for Coomassie detection) to 40 µg (maximum for complex mixtures) per well [15].
Gel Preparation and Electrophoresis Protocol
Assemble Gel Casting: Thoroughly clean glass plates with ethanol and assemble the gel casting mold with spacers [12].
Prepare Separating Gel: Mix acrylamide/bis-acrylamide solution at desired concentration (typically 8-15%), Tris-HCl buffer (pH 8.8), SDS, and water. Add ammonium persulfate (APS) and TEMED to initiate polymerization, then pour between glass plates. Overlay with water-saturated butanol or isopropanol to create a flat surface and prevent oxygen inhibition of polymerization [3] [15]. Allow to polymerize for 20-30 minutes.
Prepare Stacking Gel: After removing overlay liquid, pour stacking gel mixture (4-6% acrylamide, Tris-HCl pH 6.8, SDS, APS, and TEMED) on top of polymerized separating gel. Insert sample comb without introducing bubbles [12] [3]. Allow to polymerize for 15-20 minutes.
Set Up Electrophoresis: Mount gel in electrophoresis apparatus, fill upper and lower chambers with running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) [14] [13]. Remove air bubbles from wells using a syringe.
Run Electrophoresis: Connect power supply and run at constant voltage: 80-100 V during stacking phase, then 120-150 V during separation phase [3] [15]. Run until bromophenol blue front reaches bottom of gel (typically 45-90 minutes depending on gel size) [12].
Process Gel: Disassemble apparatus, remove gel from plates, and proceed with staining (Coomassie Blue, silver stain) or western blot transfer [12] [11].
The following workflow diagram summarizes the key steps in the denaturing SDS-PAGE protocol:
Troubleshooting and Technical Considerations
Several factors can affect the performance of SDS-PAGE and the effectiveness of SDS-mediated denaturation:
Protein Loading: Exceeding 40 µg total protein per well can cause smearing and poor resolution [15]. Complex samples may require optimization of loading amounts.
Gel Concentration: Use lower acrylamide concentrations (8-10%) for high molecular weight proteins (50-250 kDa) and higher concentrations (12-15%) for low molecular weight proteins (5-50 kDa) [12] [14]. Gradient gels (4-12% or 4-20%) provide broad separation range [3].
Heat Effects: Excessive heating during electrophoresis can cause gel warping or protein degradation [10]. Use constant voltage rather than constant current to minimize heat generation, and consider running gels in a cold room for high-current applications.
Buffer Composition: Avoid high concentrations of KCl (>200 mM) in samples as it causes SDS precipitation [15]. Dilute samples or precipitate proteins to remove interfering salts.
Alternative Methods: For applications requiring retention of protein function or metal cofactors, consider Native SDS-PAGE (reduced SDS concentration without heating) [16] or Blue Native PAGE [16], though these sacrifice some resolution.
SDS plays an indispensable role in denaturing protein gel electrophoresis by simultaneously linearizing complex protein structures and conferring a uniform charge distribution. This dual action enables researchers to separate proteins primarily by molecular weight, providing a fundamental tool for protein characterization, purity assessment, and subsequent analytical techniques. The standardized protocols and quantitative parameters presented in this application note offer researchers a reliable framework for sample preparation in denaturing electrophoresis, ensuring reproducible results across experiments and laboratories. When properly executed with attention to critical factors such as SDS concentration, heating conditions, and gel composition, SDS-PAGE remains an powerful, inexpensive, and relatively accurate method for protein separation that continues to underpin advancements in biological research and drug development.
Within the framework of denaturing protein gel electrophoresis research, the reproducibility and accuracy of results are fundamentally dependent on the initial step of sample preparation. The composition of the sample buffer is not merely a procedural formality but a critical determinant of experimental success. By systematically dismantling protein secondary, tertiary, and quaternary structures, the buffer ensures that proteins are separated solely on the basis of molecular weight [17] [18]. This application note delineates the core components of a denaturing sample buffer—the detergent, reducing agent, and buffering system—and provides detailed protocols for their use, enabling researchers to achieve high-resolution protein separation for downstream analysis in drug development and basic research.
Core Components of the Denaturing Sample Buffer
The efficacy of SDS-PAGE hinges on a sample buffer specifically formulated to denature proteins and impart a uniform charge. The following table summarizes the critical components and their primary functions.
Table 1: Critical Components of a Denaturing Sample Buffer and Their Functions
| Component | Example Agents | Primary Function | Mechanism of Action |
|---|---|---|---|
| Detergent | Sodium Dodecyl Sulfate (SDS) | Denatures proteins and imparts uniform negative charge [19] [18] | Binds to polypeptide backbone, disrupting hydrogen bonds and masking intrinsic charge; provides charge-to-mass ratio of ~1.4g SDS/g protein [17] [19]. |
| Reducing Agent | Dithiothreitol (DTT), β-mercaptoethanol (BME), Tris(2-carboxyethyl)phosphine (TCEP) | Reduces disulfide bonds [19] | Breaks covalent disulfide bonds between cysteine residues, fully linearizing polypeptides [17] [9]. |
| Buffer | Tris-HCl (pH ~6.8) | Maintains correct pH for electrophoresis [17] [19] | Provides ionic strength and optimal pH for the stacking gel in discontinuous systems, ensuring proper protein stacking [19]. |
| Density Agent | Glycerol | Adds density to the sample | Allows the sample to sink to the bottom of the gel well during loading [17] [19]. |
| Tracking Dye | Bromophenol Blue | Visualizes sample migration | Provides a visible front during electrophoresis to monitor progress [17]. |
The Role of SDS in Protein Denaturation
Sodium Dodecyl Sulfate (SDS) is the cornerstone of denaturing electrophoresis. As an anionic detergent, it performs two indispensable functions: denaturation and charge conferral. SDS disrupts hydrogen bonds and van der Waals forces that stabilize secondary and tertiary structures, effectively unfolding the protein into a random coil [18]. Concurrently, SDS molecules bind tightly to the hydrophobic regions of the polypeptide backbone in a constant ratio, approximately 1.4 grams of SDS per 1 gram of protein [19]. This coating masks the protein's intrinsic charge and imparts a uniform negative charge, ensuring that the charge-to-mass ratio is nearly identical for all proteins. This allows separation to be based primarily on molecular size within the polyacrylamide gel matrix [17] [18].
Reducing Agents: Disrupting Covalent Bonds
While SDS disrupts non-covalent interactions, it cannot break covalent disulfide bonds that stabilize tertiary and quaternary structures. Reducing agents are essential for this purpose. Agents like DTT (e.g., at 50-160 mM final concentration) or β-mercaptoethanol (e.g., 2.5% final concentration) contain thiol groups that reduce disulfide bridges (-S-S-) into free sulfhydryl groups (-SH) [17] [9]. This action completely linearizes the polypeptide, ensuring its migration accurately reflects its true molecular weight. For optimal results, reducing agents should be added fresh shortly before use, as they can oxidize and lose efficacy during storage [9] [20].
The Buffering System: Tris and pH Control
The buffer, typically Tris-HCl at pH 6.8, is critical for establishing the proper chemical environment for discontinuous gel electrophoresis [17] [19]. The pH of the sample buffer matches that of the stacking gel, which is close to the pI of glycine, the trailing ion in the running buffer. This setup creates an environment where proteins are sandwiched and concentrated into sharp zones between the leading chloride ions and the trailing glycine ions before they enter the separating gel. This stacking phenomenon is crucial for achieving high-resolution separation [19]. Furthermore, Tris helps to inhibit certain proteases, preserving sample integrity [19].
Standard Protocols for Sample Preparation
Basic Sample Denaturation Protocol
This protocol is adapted from common laboratory practices for preparing a standard protein sample for reducing SDS-PAGE [17] [20].
Materials:
- Protein sample
- 2X Laemmli Sample Buffer: 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 25% glycerol, 0.01% Bromophenol Blue [17]
- Reducing agent (e.g., 1M DTT stock or β-mercaptoethanol)
- Heating block or water bath
Method:
- Dilute Sample: Mix your protein sample with an equal volume of 2X Laemmli sample buffer. For example, combine 10 µL of protein with 10 µL of 2X buffer. If the sample is too dilute, precipitate and resuspend in 1X buffer to concentrate.
- Add Reducing Agent: Add a reducing agent to the mixture. For DTT from a 1M stock, a final concentration of 50-100 mM is typical. For β-mercaptoethanol, use a final concentration of 1-5% [17] [9].
- Heat Denature: Cap the tubes tightly and heat the samples. While traditional protocols often use 95-100°C for 5 minutes, recent evidence suggests that heating at 85°C for 2-5 minutes is sufficient for complete denaturation and can minimize protein aggregation and proteolysis [9] [20].
- Cool and Centrifuge: Briefly centrifuge the samples to collect condensation and any particulate matter.
- Load Gel: The sample is now ready to be loaded onto the polyacrylamide gel. Unused denatured samples can be stored at -20°C for short periods.
Preparation of Cell Lysates for Western Blotting
Sample preparation from cell cultures requires additional steps to solubilize proteins effectively.
Materials:
- Cell culture
- Ice-cold Phosphate-Buffered Saline (PBS)
- Lysis buffer (e.g., RIPA buffer) containing protease inhibitors
- Cell scraper (for adherent cells)
- Microcentrifuge
- 2X Laemmli Sample Buffer with reducing agent
Method:
- Harvest Cells: Place culture dish on ice. For adherent cells, wash with ice-cold PBS, then aspirate. Use a cell scraper to dislodge cells into a small volume of PBS or lysis buffer and transfer to a microcentrifuge tube. For suspension cells, pellet by centrifugation, wash with PBS, and resuspend [21].
- Lyse Cells: Add an appropriate volume of lysis buffer (e.g., 100-500 µL) containing protease inhibitors to the cell pellet. Resuspend thoroughly by pipetting and incubate on ice for 15-30 minutes.
- Clarify Lysate: Centrifuge the lysate at >12,000 rpm for 10-15 minutes at 4°C to pellet insoluble debris, including genomic DNA which can cause viscosity [9] [21].
- Prepare for Electrophoresis: Transfer the clear supernatant to a new tube. Mix an aliquot with an equal volume of 2X Laemmli sample buffer containing a reducing agent.
- Denature and Load: Heat the samples at 85°C for 2-5 minutes [9], then centrifuge. The supernatant is ready for loading onto the gel.
Diagram 1: SDS-PAGE sample preparation workflow from cell culture.
Troubleshooting and Optimization
Despite a standardized protocol, several factors can impact the quality of results. The table below outlines common issues and their solutions.
Table 2: Troubleshooting Common Sample Preparation Issues
| Problem | Potential Cause | Solution |
|---|---|---|
| Smiled or Frowning Bands | Uneven heating or current distribution; high salt concentration [9] [18]. | Ensure even heating; desalt samples via dialysis or precipitation; ensure running buffer is properly prepared. |
| Streaking or Smearing | Incomplete denaturation [22]; protein aggregation; overloading; insufficient reducing agent. | Optimize heating temperature/time; ensure fresh reducing agent; centrifuge sample before loading; reduce protein amount. |
| Multiple Bands for a Single Protein | Protease degradation; non-specific binding. | Always use protease inhibitors during lysis; keep samples on ice [21]. |
| No or Low Signal | Underloading; incomplete transfer (for WB); over-degradation. | Increase protein load; check staining protocol; confirm cell lysis efficiency. |
| Inconsistent Reduction | Oxidized reducing agent. | Prepare fresh aliquots of DTT/BME; add agent just before heating [9]. |
A key optimization is the heating temperature. While 100°C for 5 minutes is traditional, studies indicate that heating at 85°C for 2-5 minutes is sufficient for denaturation and can prevent protein aggregation and proteolysis that sometimes occurs at boiling temperatures [9]. The choice of reducing agent can also be optimized. While DTT and BME are common, TCEP is a more stable alternative that does not require preparation in a fume hood [9].
Advanced Applications: Native SDS-PAGE
A significant advancement in electrophoretic techniques is Native SDS-PAGE (NSDS-PAGE), which modifies standard conditions to preserve certain functional properties of proteins. In this approach, SDS is omitted from the sample buffer, and the heating step is eliminated. The running buffer SDS concentration is also drastically reduced (e.g., to 0.0375%) [16]. This allows for high-resolution separation while retaining enzymatic activity and non-covalently bound metal ions in many proteins, bridging the gap between fully denaturing SDS-PAGE and lower-resolution native PAGE [16]. This is particularly valuable in metalloprotein research and for analyzing functional protein complexes.
The Scientist's Toolkit
Table 3: Essential Research Reagent Solutions for Sample Preparation
| Reagent/Material | Function/Application |
|---|---|
| Laemmli Sample Buffer (2X) | Ready-to-use denaturing buffer containing Tris, SDS, glycerol, and tracking dye [17]. |
| Dithiothreitol (DTT), 1M Stock | A common reducing agent for breaking disulfide bonds. Preferred over BME for its lower odor [17]. |
| Protease Inhibitor Cocktail | Added to lysis buffers to prevent proteolytic degradation of target proteins during extraction [21]. |
| RIPA Lysis Buffer | A common detergent-based buffer for efficient lysis of mammalian cells and extraction of soluble proteins [21]. |
| Precast Polyacrylamide Gels | Offer consistency and convenience, available in various percentages and formats for optimal protein separation [20]. |
| Tris-Glycine SDS Running Buffer | The standard buffer system for discontinuous SDS-PAGE, providing the ions necessary for protein stacking and separation [20]. |
Gel electrophoresis is a foundational technique in molecular biology and proteomics for separating complex mixtures of proteins. The two primary approaches—denaturing and native gel electrophoresis—differ fundamentally in whether the protein's native structure is maintained during analysis. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) represents the most widely used denaturing method, while native PAGE (or NPAGE) preserves protein structure and function. Understanding the distinction between these techniques is critical for selecting the appropriate analytical tool for specific research objectives in drug development and basic research.
Denaturing gels like SDS-PAGE utilize ionic detergents to unfold proteins into linear chains, separating them primarily by molecular mass. In contrast, native gels maintain proteins in their folded conformation, enabling separation based on a combination of molecular size, charge, and shape. This fundamental difference dictates their respective applications, with SDS-PAGE excelling at molecular weight determination and purity assessment, while native PAGE enables functional studies and analysis of protein complexes. This application note provides a comprehensive comparison of these techniques and detailed protocols to guide researchers in selecting and implementing the optimal electrophoretic approach for their specific protein analysis needs.
Fundamental Principles and Key Differences
Mechanism of SDS-PAGE (Denaturing Electrophoresis)
SDS-PAGE operates on the principle of complete protein denaturation to achieve separation based primarily on molecular weight. The anionic detergent sodium dodecyl sulfate (SDS) plays a crucial role by binding to hydrophobic regions of proteins in a constant weight ratio (approximately 1.4 g SDS per 1 g of polypeptide), effectively shielding the protein's intrinsic charge and imparting a uniform negative charge density. This process, combined with heating at 70-100°C in the presence of reducing agents like dithiothreitol (DTT) or β-mercaptoethanol, cleaves disulfide bonds and fully denatures proteins into linear polypeptide chains. The resulting SDS-polypeptide complexes migrate through a polyacrylamide gel matrix under an electric field, with smaller proteins moving faster due to less resistance from the gel pores. The sieving effect of the cross-linked polyacrylamide gel thus separates proteins almost exclusively according to polypeptide chain length with minimal influence from compositional differences [23] [24].
Mechanism of Native PAGE
Native PAGE separates proteins under non-denaturing conditions that preserve higher-order structure, biological activity, and protein-protein interactions. Without denaturing agents, protein migration depends on both the intrinsic charge of the native protein at the running buffer pH and the molecular size and three-dimensional shape. The net charge determines migration direction and rate, while the gel matrix provides a sieving effect that regulates movement according to protein size and shape. This technique allows multimeric proteins to retain their subunit interactions, providing information about quaternary structure and enabling the recovery of enzymatically active proteins following separation. The buffer composition in native PAGE typically lacks SDS and reducing agents, and samples are not heated prior to loading to maintain structural integrity [23] [24] [25].
Comparative Analysis: SDS-PAGE vs. Native PAGE
Table 1: Key differences between SDS-PAGE and Native PAGE
| Parameter | SDS-PAGE | Native PAGE |
|---|---|---|
| Separation Basis | Molecular weight primarily | Size, charge, and shape |
| Gel Conditions | Denaturing | Non-denaturing |
| SDS Presence | Present (0.1-0.2% in buffer) | Absent |
| Reducing Agents | DTT or β-mercaptoethanol commonly used | Absent |
| Sample Preparation | Heating at 70-100°C recommended | No heating |
| Protein Charge | Uniformly negative due to SDS | Native charge maintained |
| Protein State | Denatured/unfolded | Native/folded conformation |
| Protein Function | Lost after separation | Retained after separation |
| Typical Running Temperature | Room temperature | 4°C |
| Post-Separation Recovery | Non-functional proteins | Functional proteins can be recovered |
| Primary Applications | Molecular weight determination, purity assessment, western blotting | Study of protein structure, subunit composition, functional assays, protein purification |
The critical distinction lies in the preservation of protein structure and function. While SDS-PAGE provides excellent resolution for analytical applications requiring molecular weight information, native PAGE enables researchers to study proteins in their biologically relevant state, maintaining enzymatic activity, binding capabilities, and cofactor interactions [26] [23] [24].
Application Guidelines: Selecting the Appropriate Technique
When to Use SDS-PAGE
SDS-PAGE is the method of choice for numerous applications in biochemical analysis and quality control:
- Molecular Weight Determination: The predictable relationship between migration distance and molecular size enables accurate mass estimation when appropriate standards are used [23] [24].
- Purity Assessment: The high resolution of SDS-PAGE allows detection of impurities and degradation products in protein samples [27].
- Western Blotting: Denatured proteins with linear epitopes are ideal for immunodetection following transfer to membranes [26] [23].
- Protein Expression Analysis: Comparative analysis of protein expression levels across different samples or conditions [24].
- Sample Preparation for Protein Sequencing: Denatured, reduced proteins are suitable for downstream proteomic applications [26].
When to Use Native PAGE
Native PAGE excels in applications requiring preservation of protein structure and function:
- Enzyme Activity Studies: Zymogram techniques detect enzymes based on their catalytic activity following electrophoresis [23].
- Protein-Protein Interactions: Analysis of oligomeric states and protein complexes without disrupting non-covalent bonds [26] [23].
- Binding Assays: Study of ligand-receptor interactions and cofactor binding [26].
- Purification of Active Proteins: Recovery of functional proteins for biochemical studies [23] [24].
- Analysis of Quaternary Structure: Determination of subunit composition and stoichiometry in multimeric proteins [23].
Hybrid Approach: Native SDS-PAGE (NSDS-PAGE)
A modified approach called native SDS-PAGE (NSDS-PAGE) has been developed to balance the benefits of both techniques. By removing EDTA from sample buffers, omitting the heating step, and reducing SDS concentration in the running buffer (to 0.0375%), this method maintains excellent resolution while preserving metalloprotein metal content and enzymatic activity in many cases. Research demonstrates that Zn²⁺ retention in proteomic samples increases from 26% to 98% when shifting from standard SDS-PAGE to NSDS-PAGE conditions, with seven of nine model enzymes retaining activity after separation [16].
Table 2: Buffer compositions for different electrophoretic methods
| Component | SDS-PAGE | BN-PAGE | NSDS-PAGE |
|---|---|---|---|
| Sample Buffer | 106 mM Tris HCl, 141 mM Tris Base, 0.51 mM EDTA, 2% LDS, 10% Glycerol, pH 8.5 | 50 mM BisTris, 50 mM NaCl, 10% Glycerol, pH 7.2 | 100 mM Tris HCl, 150 mM Tris Base, 0.01875% Coomassie G-250, 10% Glycerol, pH 8.5 |
| Running Buffer | 50 mM MOPS, 50 mM Tris Base, 1 mM EDTA, 0.1% SDS, pH 7.7 | Cathode: 50 mM BisTris, 50 mM Tricine, 0.02% Coomassie G-250, pH 6.8Anode: 50 mM BisTris, 50 mM Tricine, pH 6.8 | 50 mM MOPS, 50 mM Tris Base, 0.0375% SDS, pH 7.7 |
| Key Additives | SDS, EDTA | Coomassie G-250 | Reduced SDS, Coomassie G-250 |
Experimental Protocols
SDS-PAGE Protocol for Denaturing Protein Separation
Sample Preparation
- Dilution: Combine protein sample with SDS-PAGE sample buffer (typically 3:1 sample to buffer ratio). Standard SDS-PAGE sample buffer contains 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 25% glycerol, 0.01% bromophenol blue, with or without reducing agents [23] [9].
- Reduction: Add 50 mM final concentration of dithiothreitol (DTT), 2.5% β-mercaptoethanol, or 50 mM Tris(2-carboxyethyl)phosphine (TCEP) to reduce disulfide bonds [9].
- Denaturation: Heat samples at 85°C for 2-5 minutes to complete denaturation. Avoid extended heating at 100°C as this can promote proteolysis [9].
- Cooling: Briefly centrifuge heated samples to collect condensation before loading.
Gel Preparation and Electrophoresis
- Gel Selection: Choose appropriate polyacrylamide percentage based on target protein size: 8-10% for 30-200 kDa proteins, 12% for 10-100 kDa proteins, 15% for 5-60 kDa proteins. Gradient gels (e.g., 4-20%) provide broader separation range [23].
- Apparatus Setup: Assemble gel electrophoresis unit and fill with running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) [23].
- Loading: Pipette 10-50 μg protein per lane for Coomassie staining or 1-10 μg for silver staining. Include molecular weight markers in one lane.
- Electrophoresis Conditions:
- Initial stacking phase: 50-60 V constant voltage until dye front enters resolving gel (~30 minutes)
- Separation phase: 100-150 V constant voltage until dye front approaches gel bottom (45-90 minutes)
- For large format gels: 200-300 V may be appropriate [28]
- Temperature Management: Run at room temperature. For constant current settings, consider ice bath or cold room to prevent "smiling" artifacts from heat buildup [28].
Native PAGE Protocol for Native Protein Separation
Sample Preparation
- Buffer Compatibility: Ensure sample buffer has low ionic strength (<50 mM NaCl) and compatible pH (typically 6.8-8.5) to prevent precipitation and artifacts [9].
- Non-Denaturing Conditions: Mix sample with native sample buffer (50-100 mM Tris-HCl, 10% glycerol, trace dye such as bromophenol blue, pH 6.8). Do not add SDS or reducing agents [23] [24].
- No Heating: Maintain samples at 4°C throughout preparation to preserve native structure [24].
Gel Preparation and Electrophoresis
- Gel Composition: Prepare polyacrylamide gels without SDS. Both resolving gel (typically 6-10%) and stacking gel (4-5%) use Tris-HCl buffers at appropriate pH (resolving gel: pH 8.8; stacking gel: pH 6.8) [23] [25].
- Running Buffer: Use Tris-glycine (25 mM Tris, 192 mM glycine, pH 8.3-8.8) without SDS [23].
- Loading: Apply samples as in SDS-PAGE but include native molecular weight standards if available.
- Electrophoresis Conditions:
- Run at constant voltage (100-150 V) with cooling
- Maintain temperature at 4°C using cooled circulation or run in cold room [24]
- Continue until dye front reaches gel bottom (typically 60-90 minutes)
- Post-Electrophoresis Processing: For activity assays, proceed immediately to staining or transfer. Proteins can be recovered by passive diffusion or electroelution for functional studies [23].
Visualization and Decision Framework
The following decision diagram illustrates the key factors in selecting between denaturing and native gel electrophoresis approaches:
Research Reagent Solutions
Table 3: Essential reagents for protein gel electrophoresis
| Reagent/Category | Function/Purpose | Specific Examples |
|---|---|---|
| Detergents | Denature proteins and impart charge | Sodium dodecyl sulfate (SDS) for denaturing gels [23] |
| Reducing Agents | Break disulfide bonds | Dithiothreitol (DTT), β-mercaptoethanol, Tris(2-carboxyethyl)phosphine (TCEP) [9] |
| Gel Matrix Components | Form porous sieving matrix | Acrylamide, bis-acrylamide (cross-linker), ammonium persulfate (APS, initiator), TEMED (catalyst) [23] |
| Buffer Systems | Maintain pH and conductivity | Tris-glycine, Tris-HCl, MOPS, Bis-Tris [23] [16] |
| Tracking Dyes | Visualize migration front | Bromophenol blue, Coomassie G-250, Phenol Red [16] [29] |
| Molecular Weight Standards | Reference for size determination | Pre-stained or unstained protein ladders with known molecular weights [23] |
| Staining Reagents | Visualize separated proteins | Coomassie Brilliant Blue, silver stain, SYPRO Ruby, SimplyBlue SafeStain [27] [23] |
The choice between denaturing SDS-PAGE and native PAGE represents a critical decision point in experimental design for protein analysis. SDS-PAGE remains the gold standard for determining molecular weight, assessing sample purity, and preparing samples for western blotting, while native PAGE enables researchers to probe protein function, complex formation, and tertiary structure. The recent development of NSDS-PAGE offers a promising intermediate approach that maintains high resolution while preserving some functional characteristics. By understanding the principles, applications, and methodological requirements of each technique outlined in this application note, researchers can make informed decisions to optimize their protein separation strategies for specific research objectives in drug development and basic science.
The Critical Impact of Sample Integrity on Final Data Quality and Reproducibility
The reliability of any scientific data generated from denaturing protein gel electrophoresis is fundamentally dependent on the quality of the starting material. Sample integrity serves as the cornerstone of experimental reproducibility, particularly in drug development where regulatory compliance and analytical validation are paramount [30]. Degraded or compromised samples introduce significant variability that can invalidate experimental results, waste valuable resources, and lead to erroneous scientific conclusions.
The preparation of samples for denaturing gel electrophoresis presents unique challenges for maintaining integrity. Proteins are susceptible to proteolytic degradation, post-translational modifications, aggregation, and denaturation during isolation and purification. Unlike nucleic acids, which have well-established integrity metrics like the RNA Integrity Number (RIN) [31], protein integrity assessment often requires multiple complementary approaches. This application note examines the critical relationship between sample integrity and data quality, provides validated methodologies for integrity assessment, and establishes best practices to ensure reproducible results in electrophoretic analyses.
The Fundamental Link Between Sample Integrity and Data Quality
Consequences of Sample Degradation
The integrity of a biological sample directly determines the accuracy, reliability, and interpretability of electrophoretic data. Compromised sample integrity manifests in several characteristic ways on denaturing gels:
- Protein Degradation: Proteolytic cleavage results in the appearance of multiple unexpected lower-molecular-weight bands, smearing, or the disappearance of target protein bands [32]. This degradation occurs when protease inhibitors are ineffective or there is excessive delay between cell lysis and sample denaturation.
- Improper Folding and Aggregation: Incomplete denaturation leads to aberrant migration patterns, high-molecular-weight aggregates stuck in the well, or inconsistent banding between replicates [32].
- Post-Translational Modifications: Unintended modifications during sample preparation can cause shifts in molecular weight or altered charge states that affect migration.
The analytical validation guidance from regulatory agencies like the FDA emphasizes that test methods must demonstrate specificity for the target analyte, which can be severely compromised by sample degradation [30]. For protein electrophoresis, this specificity is reflected in the ability to clearly resolve the target protein from degradation products and contaminants.
Quantitative Impact of Sample Integrity on Data Reproducibility
The relationship between sample integrity and experimental outcomes can be quantified across multiple parameters. The following table summarizes key integrity indicators and their impact on data interpretation:
Table 1: Quantitative Impact of Sample Integrity on Electrophoresis Data
| Integrity Parameter | High-Quality Indicator | Compromised Indicator | Impact on Data Interpretation |
|---|---|---|---|
| Protein Band Sharpness | Distinct, sharp bands | Diffuse or smeared bands | Reduced accuracy in molecular weight determination |
| Background Signal | Low background | High background throughout lane | Obscured target bands; impaired quantification |
| Inter-experiment Consistency | <10% CV in band intensity | >25% CV in band intensity | Compromised reproducibility and statistical power |
| Degradation Products | Minimal to no secondary bands | Multiple lower-weight bands | Inaccurate quantification of target protein |
| Aggregation State | Minimal material in well | Significant high-weight aggregation | Altered functional interpretation of protein state |
The critical nature of sample integrity is further emphasized by journal policies, such as those from Nature Portfolio, which require authors to submit unprocessed original data for gels and western blots and maintain strict guidelines against inappropriate image manipulation that might obscure integrity issues [33].
Establishing Sample Integrity Metrics for Denaturing Gel Electrophoresis
Pre-electrophoresis Integrity Assessment
Prior to gel analysis, several quality control checkpoints can predict electrophoretic performance:
- Spectrophotometric Purity Assessment: The A260/A280 ratio provides a preliminary indication of protein purity, with ideal ratios typically between 1.8-2.0, though this varies by protein [34]. Significant deviation may indicate nucleic acid contamination.
- Protein Quantification Accuracy: Consistent results across multiple quantification methods (e.g., Bradford, BCA) suggests sample integrity, while significant discrepancies indicate potential interference from contaminants [32].
- Visual Inspection: High-quality protein precipitates after TCA precipitation appear as compact pellets, while diffuse pellets may indicate degradation.
Electrophoretic Integrity Indicators
The electrophoretic separation itself provides the most direct assessment of protein integrity:
- Band Pattern Consistency: Intact proteins demonstrate reproducible banding patterns across replicates, while degraded samples show variable patterns.
- Signal-to-Noise Ratio: High-integrity samples yield strong target band signals with minimal background [32].
- Molecular Weight Verification: Migration consistent with expected molecular weight confirms proper denaturation and absence of significant proteolysis.
Experimental Protocols for Sample Integrity Assessment
Protocol: Comprehensive Protein Integrity Evaluation for Denaturing Gel Electrophoresis
Principle: This protocol provides a systematic approach to prepare and evaluate protein samples to ensure they maintain integrity throughout the process of denaturing gel electrophoresis.
Materials:
- Lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS)
- Protease inhibitor cocktail (add fresh)
- BCA or Bradford protein assay kit
- 2X Laemmli sample buffer (4% SDS, 20% glycerol, 120 mM Tris-HCl, pH 6.8, 0.02% bromophenol blue)
- β-mercaptoethanol or DTT
- Precast or homemade polyacrylamide gels
- Electrophoresis system and power supply
Procedure:
- Cell Lysis and Protein Extraction
- Aspirate media from cultured cells and wash with ice-cold PBS.
- Add appropriate volume of lysis buffer with freshly added protease inhibitors (e.g., 1 mM PMSF, 1 μg/mL leupeptin, 1 μg/mL aprotinin).
- Incubate on ice for 30 minutes with occasional gentle vortexing.
- Clarify lysate by centrifugation at 14,000 × g for 15 minutes at 4°C.
- Transfer supernatant to a fresh pre-chilled tube.
Protein Quantification and Purity Assessment
- Dilute sample 1:10 and 1:20 in lysis buffer.
- Perform protein assay in triplicate according to manufacturer's protocol.
- Measure A260/A280 ratio for key samples to assess nucleic acid contamination.
- Record concentration and purity ratios.
Sample Preparation for Denaturing Electrophoresis
- Aliquot appropriate volume of protein extract (typically 20-50 μg) to fresh tubes.
- Add equal volume of 2X Laemmli sample buffer.
- Add reducing agent (final concentration: 100 mM DTT or 5% β-mercaptoethanol).
- Heat samples at 95°C for 5-10 minutes.
- Briefly centrifuge to collect condensation.
Electrophoretic Separation and Integrity Assessment
- Load samples and molecular weight markers onto polyacrylamide gel.
- Conduct electrophoresis at constant voltage (e.g., 100-120V for mini-gels) until dye front reaches bottom.
- Process gel for staining (Coomassie, silver) or transfer for western blotting.
Integrity Scoring and Documentation
- Image gel under standardized conditions.
- Evaluate band sharpness, background, and presence of degradation products.
- Assign integrity score based on predefined criteria (see Table 1).
- Document all observations and anomalies.
Troubleshooting:
- Protein Degradation: Ensure protease inhibitors are fresh; maintain samples on ice; minimize freeze-thaw cycles.
- Poor Band Resolution: Check sample preparation; ensure adequate denaturation; verify gel composition.
- High Background: Optimize washing steps; check antibody specificity (for western blotting); verify reagent quality.
Protocol: Validation of Electrophoretic Methods for Integrity Assessment
Principle: Based on FDA's Analytical Test Method Validation guidance [30], this protocol establishes a framework for validating electrophoretic methods to ensure they reliably detect sample integrity issues.
Materials:
- Reference standard protein of known integrity
- Intentionally degraded protein samples
- Electrophoresis system and imaging equipment
- Image analysis software
Procedure:
- Define Method Purpose and Critical Parameters
- Clearly state the method's purpose: to evaluate protein integrity for denaturing gel electrophoresis.
- Identify critical parameters: band sharpness, molecular weight accuracy, absence of degradation products.
Establish Specificity/Selectivity
- Analyze reference standard alongside intentionally degraded samples.
- Demonstrate method's ability to distinguish intact vs. degraded protein.
- Show absence of interference from buffer components or contaminants.
Determine Range
- Establish the range of protein concentrations over which integrity can be reliably assessed.
- Typically 10-100 μg for Coomassie staining; 1-20 μg for silver staining.
Assess Accuracy and Precision
- Prepare samples of known integrity status (confirmed by multiple methods).
- Evaluate method's ability to correctly classify samples as intact or compromised.
- Determine repeatability (within-run) and intermediate precision (between-run, between-days) using control samples.
Document Robustness
- Test deliberate variations in method parameters (e.g., heating time, sample loading).
- Establish stability of samples and reagents under defined storage conditions.
Validation Acceptance Criteria:
- Specificity: Method must clearly differentiate intact from degraded samples.
- Precision: CV of band intensity measurements <15% for repeatability.
- Range: Linear correlation between load and signal (R² > 0.95) across working range.
Workflow for Maintaining Sample Integrity
The process of ensuring sample integrity requires a systematic approach from sample collection through data analysis. The following workflow outlines the critical control points:
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 2: Essential Reagents for Maintaining Sample Integrity in Denaturing Gel Electrophoresis
| Reagent Category | Specific Examples | Function in Integrity Maintenance | Quality Control Indicators |
|---|---|---|---|
| Protease Inhibitors | PMSF, leupeptin, aprotinin, complete protease inhibitor cocktails | Prevent proteolytic degradation during and after cell lysis | Consistent banding patterns; absence of degradation products |
| Lysis Buffers | RIPA buffer, NP-40 based buffers, SDS-containing buffers | Efficient extraction while maintaining protein solubility | High yield; minimal aggregation; clear solutions |
| Detergents | SDS, Triton X-100, CHAPS | Solubilize membrane proteins; maintain denatured state | Proper migration; minimal smearing |
| Reducing Agents | DTT, β-mercaptoethanol, TCEP | Break disulfide bonds; ensure complete unfolding | Consistent mobility; elimination of higher-order structures |
| Denaturing Agents | Urea, thiourea, SDS | Unfold proteins; inactivate enzymes | Sharp band resolution; accurate molecular weight |
| Protein Assays | BCA, Bradford, Lowry | Accurate quantification for equal loading | Linear standard curves; consistent inter-assay results |
| Staining Reagents | Coomassie Brilliant Blue, silver nitrate, SYPRO Ruby | Detect proteins with high sensitivity and linear dynamic range | Low background; specific staining without precipitation |
Sample integrity stands as the fundamental determinant of data quality and reproducibility in denaturing protein gel electrophoresis. Through implementation of systematic integrity assessment protocols, adherence to validated methodologies, and vigilant quality control at each stage of sample preparation, researchers can ensure the generation of reliable, interpretable, and reproducible data. The framework presented in this application note provides laboratory scientists with the tools necessary to establish and maintain sample integrity, thereby strengthening the foundation of electrophoretic analysis in biomedical research and drug development.
A Step-by-Step Protocol for Flawless Denaturing Protein Sample Preparation
Sample preparation is the foundational step in denaturing protein gel electrophoresis research, directly determining the success and reproducibility of downstream analyses. The critical process of cell lysis and tissue homogenization must accomplish complete disruption of cellular structures to release proteins while maintaining their integrity for accurate separation and detection. This application note provides a structured framework for selecting appropriate lysis buffers and methods tailored to specific experimental requirements within denaturing electrophoresis workflows. By integrating both established protocols and recent comparative efficiency data, we offer researchers a comprehensive guide to optimizing this crucial initial phase of protein analysis.
Understanding Lysis Buffer Composition and Selection
The selection of an appropriate lysis buffer depends primarily on the cellular localization of the target protein and the compatibility with downstream denaturing gel electrophoresis. Denaturing electrophoresis, particularly SDS-PAGE, relies on the complete unfolding of proteins and uniform coating with sodium dodecyl sulfate (SDS) to separate polypeptides based on molecular weight rather than native charge or structure [27] [35]. The buffer must therefore effectively disrupt protein-protein interactions, solubilize target proteins, and inactivate cellular proteases and phosphatases that could degrade the sample.
Table 1: Lysis Buffer Recommendations for Different Protein Localizations
| Target Protein Location | Recommended Buffer Type | Key Components | Compatibility Notes |
|---|---|---|---|
| Whole Cell (mild extraction) | Mild, non-ionic detergent-based [36] | 25 mM bicine, pH 7.6; Non-ionic detergent [36] | Retains protein-protein interactions; may require optimization for SDS-PAGE |
| Whole Cell (membrane-bound, nuclear) | RIPA Buffer [36] | 25 mM Tris-HCl, 150 mM NaCl, 1% NP-40/Triton X-100, 1% sodium deoxycholate, 0.1% SDS [36] | Effective for difficult-to-solubilize proteins; compatible with denaturing gels |
| Cytoplasmic | NP-40 Lysis Buffer [36] | 50 mM Tris, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1% NP-40 [36] | Ideal for soluble cytoplasmic proteins; compatible with electrophoresis |
| Membrane Proteins | SDS-based Buffer [37] | 1-4% SDS [37] | Most effective for solubilizing membrane proteomes; requires SDS removal (e.g., SP3) for MS analysis |
| Chaotropic Agent-based | Guanidinium HCl or Urea Buffer [37] [38] | Guanidinium HCl or 8 M Urea, CHAPS, DTE [37] [38] | Strong denaturation; GnHCl is LC-MS compatible; Urea/CHAPS suitable for 2DE |
The inclusion of protease and phosphatase inhibitors is critical in all lysis buffers to prevent artificial proteolysis and maintain post-translational modification states [36]. For denaturing electrophoresis, the anionic detergent SDS is a key component, binding to proteins in a constant mass ratio (1.4:1) and imparting a uniform negative charge essential for separation by molecular weight [35]. The reducing agents dithiothreitol (DTT) or β-mercaptoethanol are added to break disulfide bonds, ensuring complete protein unfolding [35].
Quantitative Comparison of Lysis Buffer Efficiency
Recent systematic comparisons provide quantitative data on lysis buffer efficiency, particularly for challenging sample types. A 2022 study directly compared SDS and guanidinium hydrochloride (GnHCl) buffers for proteomic analysis of human cells and plasma using different preparation workflows [37].
Table 2: Quantitative Performance of Lysis Buffers and Preparation Methods in HeLa Cells
| Lysis Buffer | Preparation Method | Number of Quantified Proteins (Mean ± SEM) | Number of Quantified Peptides (Mean ± SEM) | Peptides with Zero Missed Cleavages (%) |
|---|---|---|---|---|
| SDS-based Buffer | SP3 | 6131 ± 20 | 47,088 ± 345 | 84.6% |
| GnHCl-based Buffer | SP3 | 5895 ± 37 | 48,940 ± 345 | 77.5% |
| GnHCl-based Buffer | In-Solution Digestion (ISD) | 4851 ± 44 | 40,505 ± 630 | 38.0% |
This data demonstrates that the combination of SDS-based lysis with the SP3 (single-pot, solid-phase-enhanced sample preparation) method yields the highest number of quantified proteins while maintaining excellent digestion efficiency, as evidenced by the high percentage of peptides with zero missed cleavages [37]. The SP3 method effectively removes SDS, which would otherwise interfere with downstream enzymatic steps and chromatography [37].
For specialized tissues, buffer selection significantly impacts protein recovery profiles. A 2024 study on muscle tissue compared SDS-based and Urea/CHAPS-based extraction methods for two-dimensional gel electrophoresis [38]. The SDS-based method (Method A: 2% SDS, 1% DTE) involved homogenization followed by heat denaturation and acetone precipitation, while the Urea/CHAPS method (Method B: 8 M Urea, 4% CHAPS, 1% DTE, 40 mM Tris) omitted the precipitation step [38]. The Urea/CHAPS method yielded a higher mean number of total protein spots, though the SDS method demonstrated superior extraction of proteins with specific chemical-physical characteristics [38]. This highlights that parallel application of complementary extraction methods can provide more comprehensive proteomic profiling of complex tissues [38].
Detailed Experimental Protocols
Protocol for Adherent Mammalian Cell Lysis
This protocol is optimized for obtaining whole-cell lysates from adherent cultures for denaturing SDS-PAGE [36] [35].
Materials:
- Ice-cold Phosphate-Buffered Saline (PBS)
- Appropriate ice-cold lysis buffer (see Table 1) with freshly added protease/phosphatase inhibitors
- Cell scraper
- Pre-cooled microcentrifuge tubes
- Microcentrifuge
Procedure:
- Place the cell culture dish on ice and carefully remove the culture medium.
- Wash cells gently with ice-cold PBS to remove residual media and serum proteins.
- Aspirate PBS completely and add ice-cold lysis buffer (~1 mL per 10⁷ cells or 100 mm plate; ~200-400 µL for a 6-well plate) [36].
- Scrape adherent cells off the dish using a cold plastic cell scraper and transfer the suspension to a pre-cooled microcentrifuge tube.
- Maintain constant agitation for 30 minutes at 4°C to ensure complete lysis [35].
- Centrifuge the lysate at ~14,000 × g for 15 minutes at 4°C to pellet insoluble cell debris [36].
- Transfer the supernatant (clarified lysate) to a new pre-cooled tube. Discard the pellet.
- Determine protein concentration using a compatible assay (e.g., BCA or Bradford assay) before proceeding to sample preparation for electrophoresis.
Protocol for Tissue Homogenization and Lysis
This protocol is designed for protein extraction from animal or plant tissues, which present additional challenges like tough extracellular matrices and interfering compounds [36] [39].
Materials:
- Liquid nitrogen
- Mortar and pestle (pre-cooled) or electric homogenizer with cooled chamber
- Ice-cold lysis buffer with protease/phosphatase inhibitors
- Refrigerated microcentrifuge
Procedure:
- Dissect the tissue of interest with clean tools as quickly as possible on ice to minimize proteolysis.
- For storage, snap-freeze the tissue by immersing it in liquid nitrogen and store at -80°C. For immediate processing, keep the tissue on ice.
- For frozen tissue, pulverize it using a mortar and pestle pre-cooled with liquid nitrogen.
- Add ice-cold lysis buffer to the powdered tissue (~300 µL per ~5 mg tissue). The optimal ratio is approximately 50 mg tissue to 1,000 µL of lysis buffer, which can be adjusted for more concentrated extracts [36] [35].
- Homogenize the mixture using an electric homogenizer. Maintain the sample on ice during and between homogenization bursts to prevent heating.
- Rinse the homogenizer blade with additional lysis buffer to recover the entire sample.
- Incubate the homogenate with constant agitation for 2 hours at 4°C (e.g., on an orbital shaker in a cold room) to ensure complete extraction [35].
- Centrifuge at 10,000 × g for 5-20 minutes at 4°C to pellet tissue debris [36] [39].
- Transfer the supernatant to a fresh tube kept on ice. The supernatant is the protein extract ready for quantification.
Sample Preparation for Denaturing Gel Electrophoresis
After lysis and quantification, proteins must be denatured and reduced for SDS-PAGE.
Materials:
- SDS sample buffer (e.g., 2X or 4X Laemmli buffer: 4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue, 0.125 M Tris HCl, pH 6.8) [35]
- Heating block or water bath
Procedure:
- Mix the protein lysate with an equal volume of 2X Laemmli sample buffer (for a final concentration of 1X). Scale volumes according to well capacity.
- For reduced samples, the buffer contains DTT or β-mercaptoethanol to break disulfide bonds. Omit these for non-reduced analysis [35].
- Vortex the mixture thoroughly.
- Heat the samples at 70°C for 2-10 minutes or at 95-100°C for 5 minutes. The lower temperature is recommended to prevent proteolysis and aggregation of multi-pass membrane proteins [36] [35].
- Briefly centrifuge the tubes to collect condensation and ensure the entire sample is at the bottom of the tube.
- The samples are now ready to be loaded onto an SDS-polyacrylamide gel.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Reagents for Cell Lysis and Denaturing Electrophoresis
| Reagent Category | Specific Examples | Function and Application Notes |
|---|---|---|
| Detergents | SDS, NP-40, Triton X-100, CHAPS, Sodium Deoxycholate [36] [37] [38] | Solubilize membranes and proteins; SDS provides strong denaturation for electrophoresis. |
| Chaotropic Agents | Urea, Guanidinium Hydrochloride (GnHCl) [37] [38] | Disrupt hydrogen bonding and protein structure; GnHCl is MS-compatible. |
| Reducing Agents | Dithiothreitol (DTT), β-Mercaptoethanol, Dithioerythritol (DTE) [38] [35] | Break disulfide bonds for complete protein unfolding in denaturing gels. |
| Protease Inhibitors | PMSF, Commercial Cocktails (e.g., Halt, Pierce) [36] [39] | Prevent protein degradation during and after lysis; use broad-spectrum cocktails. |
| Phosphatase Inhibitors | Sodium Fluoride (NaF), Sodium Orthovanadate (Na3VO4) [36] | Preserve phosphorylation states by inhibiting cellular phosphatases. |
| Protein Assays | BCA, Bradford Assay [36] [35] | Quantify protein concentration for equal loading across gel lanes. |
| Sample Buffer | Laemmli Buffer (SDS, Glycerol, Bromophenol Blue, Tris, Reducing Agent) [35] | Denature proteins and provide density for gel loading; contains tracking dye. |
Optimal cell lysis and tissue homogenization require a strategic choice of buffer composition and extraction methodology aligned with the target protein's characteristics and downstream analytical application. For denaturing gel electrophoresis, SDS-based buffers provide robust protein solubilization and denaturation, while chaotropic agents like urea and GnHCl offer effective alternatives, particularly for mass spectrometry-compatible workflows. The integration of protease inhibitors and efficient mechanical disruption ensures the recovery of intact, representative protein populations. By applying the principles and protocols outlined in this document, researchers can standardize and optimize this critical first step in protein analysis, establishing a solid foundation for reliable and reproducible electrophoretic separation.
The integrity of protein samples is the foundation of reliable data in denaturing protein gel electrophoresis. Proteolytic degradation during sample preparation can artifactually alter protein molecular weights, obscure true banding patterns, and ultimately compromise experimental conclusions. Protease inhibitor cocktails represent a critical first-line defense against these artifacts, preserving the native protein population from endogenous proteases released upon cell lysis. Within the context of sample preparation for electrophoretic analysis, these chemical additives are not merely optional but essential components for ensuring that observed results reflect biological reality rather than preparation artifacts. This application note details the strategic implementation of protease inhibitor cocktails to maintain sample integrity throughout the preparation workflow, providing specific protocols suitable for both cell culture and tissue samples.
Background and Principles
The Problem of Proteolytic Degradation
Upon cell lysis, proteins become immediately vulnerable to degradation by a spectrum of endogenous proteases. Serine, cysteine, aspartic, and metallo-proteases are released from cellular compartments, and their combined activity can rapidly degrade proteins of interest. This degradation is particularly problematic for denaturing gel electrophoresis, where it can manifest as smearing bands, unexpected lower molecular weight bands, or the complete absence of bands for target proteins. The use of specific protease inhibitors, combined into broad-spectrum cocktails, effectively neutralizes this threat by simultaneously targeting multiple protease classes.
Role in the Western Blot Workflow
Protease inhibitor cocktails are integral to the initial sample preparation stage of the western blot protocol [40]. Their function is to stabilize the protein population immediately upon lysis, ensuring that the protein separation patterns observed after SDS-PAGE accurately represent the in vivo state. This stabilization is crucial for all subsequent analysis, including protein quantification, immunodetection, and data interpretation. Without effective protease inhibition, the fundamental principle of western blotting—correlating band identity and intensity with specific protein presence and abundance—becomes unreliable.
Research Reagent Solutions
The following toolkit outlines essential reagents required for effective protease inhibition during sample preparation.
Table 1: Essential Research Reagent Toolkit for Protease Inhibition
| Reagent | Function & Application |
|---|---|
| Protease Inhibitor Cocktail | A premixed combination of inhibitors targeting multiple protease classes (e.g., serine, cysteine, aspartic, metallo-proteases). Added to lysis buffer to prevent protein degradation during and after cell/tissue disruption [40]. |
| Phosphatase Inhibitor Cocktail | An essential additive for preserving post-translational modifications, particularly phosphorylated residues on proteins. Used in conjunction with protease inhibitors when studying phosphoproteins [40]. |
| Lysis Buffer (e.g., RIPA) | A detergent-based buffer used to solubilize cells and tissues, releasing protein content. The chemical foundation into which protease inhibitors are added [40]. |
| Dithiothreitol (DTT) | A reducing agent included in the loading buffer to break disulfide bonds in proteins, ensuring complete denaturation and linearization for accurate size-based separation [40]. |
Recommended Protocols
Protocol A: Sample Preparation from Cell Culture
This protocol is adapted from comprehensive western blot procedures and is designed for adherent or suspension mammalian cells [40].
Materials
- Cell culture sample
- Ice-cold PBS
- Lysis Buffer (e.g., RIPA buffer)
- Protease Inhibitor Cocktail
- Phosphatase Inhibitor Cocktail (if studying phosphorylated proteins)
- Dithiothreitol (DTT)
- Loading Buffer
- BCA or Bradford Assay Kit
Procedure
- Prepare Lysis Buffer: Add protease inhibitor cocktail (and phosphatase inhibitors if needed) to ice-cold lysis buffer immediately before use [40].
- Harvest Cells:
- For adherent cells, wash the monolayer with ice-cold PBS. Scrape cells into fresh PBS and pellet by centrifugation (100–500 x g, 5 min, 4°C).
- For suspension cells, pellet by centrifugation (100–500 x g, 5 min, 4°C) and wash twice with ice-cold PBS.
- Lyse Cells: Resuspend the cell pellet in ice-cold lysis buffer (recommended: ~1 mL per 1x10^7 cells). Incubate the suspension for 10 minutes at 4°C with gentle rocking.
- Sonicate: Sonicate the suspension on ice to ensure complete cell disruption. Optimize time and intensity for your instrument.
- Clarify Lysate: Centrifuge the lysate at 14,000–17,000 x g for 5 minutes at 4°C. Transfer the supernatant (the soluble protein lysate) to a fresh tube placed on ice. Discard the insoluble pellet.
- Determine Protein Concentration: Use a BCA or Bradford assay to determine the protein concentration of the clarified lysate.
- Prepare Sample for Storage: Dilute the lysate with loading buffer containing DTT to a final concentration of 1–2 mg/mL. Boil the samples at 100°C for 10 minutes to fully denature proteins. Samples can now be used immediately for gel electrophoresis or stored at -80°C [40].
Protocol B: Sample Preparation from Tissue
Tissue samples present a greater challenge due to higher protease content and density. The protocol below is optimized for this application [40].
Materials
- Tissue sample
- Tools for dissection (on ice)
- Lysis Buffer with Protease/Phosphatase Inhibitors
- Glass bead tubes
- Automated homogenizer
- BCA or Bradford Assay Kit
- Loading Buffer with DTT
Procedure
- Dissect Tissue: Rapidly dissect the tissue of interest on ice using clean tools to minimize protease activity.
- Homogenize: Place the dissected tissue (e.g., 200 mg) into a tube containing ice-cold lysis buffer (e.g., 1200 µL) and glass beads. Homogenize using an automated homogenizer for approximately 3 minutes at 4°C. Pause halfway to avoid overheating. Incubate the homogenate for 5 more minutes on ice.
- Clarify Lysate: Centrifuge the homogenate at 14,000–17,000 x g for 5–10 minutes at 4°C. Transfer the supernatant (cleared lysate) to a fresh tube.
- Determine Protein Concentration: Quantify the protein concentration using a BCA or Bradford assay.
- Prepare for Storage: Aliquot the lysate and dilute with loading buffer containing DTT. Boil at 100°C for 10 minutes. The samples are now ready for SDS-PAGE or can be stored at -80°C [40].
Experimental Workflow and Data Presentation
The following diagram and tables summarize the key stages and considerations for successful sample preparation.
Diagram 1: Sample preparation workflow with key stages for protease inhibition.
Quantitative Guidelines for Sample Loading
To achieve optimal resolution and avoid artifacts, loading the correct amount of protein is crucial. The following table provides recommended loading quantities for different sample types.
Table 2: Recommended Protein Load for Denaturing Gels
| Sample Type | Recommended Load | Critical Note |
|---|---|---|
| Whole Cell/Tissue Lysate | 10–40 µg of total protein | Prevents well overloading, which can cause smearing and cross-well contamination [40]. |
| Purified Protein | 10–500 ng | Amount varies significantly based on protein abundance and detection method sensitivity. |
Inhibitor Selection Guide
Different experimental aims require inhibition of specific protease classes. The table below outlines common inhibitor targets.
Table 3: Guide to Protease Inhibitor Types
| Inhibitor Target | Recommended Use Case |
|---|---|
| Broad-Spectrum Proteases | Standard preparation of total protein lysates for general western blotting [40]. |
| Phosphatases | Essential when analyzing protein phosphorylation states to prevent loss of phospho-epitopes [40]. |
The integration of protease inhibitor cocktails into sample preparation protocols is a non-negotiable step for generating robust and reproducible data in denaturing protein gel electrophoresis. By halting proteolytic activity at the moment of cell disruption, these additives preserve the true molecular weight and integrity of proteins, which is the cornerstone of accurate interpretation of electrophoretic banding patterns. The protocols and guidelines provided here offer researchers a reliable framework for preparing high-quality samples, thereby ensuring that the data generated reflects genuine biology rather than preparation artifacts.
Within the broader context of sample preparation for denaturing protein gel electrophoresis research, the steps of denaturation and reduction are critical for obtaining reliable, reproducible, and high-resolution results. These processes ensure that proteins are unfolded and their disulfide bonds are cleaved, allowing separation strictly by molecular weight during SDS-PAGE. The selection of an appropriate reducing agent and the application of correct heating conditions are fundamental to the success of downstream applications, from routine western blotting to advanced proteomic analyses. This application note provides a detailed, evidence-based guide to optimizing these key steps, focusing on a comparative analysis of the most common reducing agents: dithiothreitol (DTT), β-mercaptoethanol (BME), and tris(2-carboxyethyl)phosphine (TCEP).
Reducing Agent Selection: A Comparative Analysis
The primary function of a reducing agent in protein sample preparation is to break disulfide bonds between cysteine residues. This action ensures that multi-subunit proteins are separated into their individual polypeptides and that folded proteins are completely linearized by SDS. The choice of reductant can influence experimental outcomes through its chemical stability, strength, and compatibility with downstream techniques.
Table 1: Comparison of Common Protein Reducing Agents
| Property | DTT | BME | TCEP |
|---|---|---|---|
| Chemical Class | Thiol-based | Thiol-based | Phosphine-based |
| Mechanism | Thiol-disulfide exchange; forms a stable cyclic disulfide [41] | Thiol-disulfide exchange; forms an intermolecular disulfide dimer | Direct reduction via phosphine; irreversibly forms phosphine oxide [42] |
| Reducing Power | Strong | Moderate | Very Strong [42] [41] |
| Odor | Low | Strong, unpleasant [42] | Nearly odorless [42] |
| Stability in Buffer | Prone to air oxidation; stock solutions require aliquoting and freezing [41] | Prone to air oxidation | Highly stable in aqueous solution; resistant to air oxidation [42] |
| Effective pH Range | Most effective at pH >7 [41] | Effective at neutral to basic pH | Effective across a broad pH range (1.5 - 8.5) [42] |
| Thiol-Free | No | No | Yes [42] [43] |
| Compatibility with Maleimides | Incompatible (quenches thiol-reactive reagents) [42] | Incompatible | Compatible (preferred for cysteine labeling) [42] |
| Interference with IMAC | Can interfere (reacts with metal ions) [42] | Can interfere (reacts with metal ions) [42] | Minimal interference [42] |
| Membrane Permeability | Permeable | Permeable | Impermeable [42] |
| Typical Working Concentration | 1-100 mM [44] [41] | 0.1-1% (v/v) | 0.1-1.0 mM [42] |
Key Selection Guidelines
- For most general applications: DTT is a robust and powerful choice, offering a good balance of strong reducing power and low odor. Its main drawback is the need to prepare fresh stock solutions frequently or store them frozen to prevent oxidation [41].
- When stability is a priority: TCEP is superior, as it does not oxidize in air. It is the reagent of choice for experiments requiring long-term stability of the reducing environment, for work at low pH, or for any downstream step involving thiol-reactive chemistry like maleimide-based labeling [42].
- As a historical alternative: BME is effective but is used less frequently today due to its noxious odor and lower reducing power compared to DTT and TCEP [42].
Optimal Heating Conditions for Sample Denaturation
After mixing the protein sample with SDS-containing sample buffer and a reducing agent, heating is the final critical step to achieve complete denaturation. The goal is to fully unfold the protein, facilitating uniform SDS coating to impart a negative charge.
A common misconception is that samples must be boiled (100°C). However, excessive heating can lead to protein aggregation, proteolysis, or modification of sensitive epitopes [36]. Based on optimized western blot protocols, the recommended condition is heating at 70°C for 2–10 minutes [36]. This provides sufficient thermal energy to denature the vast majority of proteins without the negative effects associated with boiling.
Table 2: Protocol for Denaturation and Reduction of Protein Samples
| Step | Parameter | Recommendation | Rationale |
|---|---|---|---|
| 1. Sample Mixing | Sample Buffer Composition | Final concentration of 1X SDS/LDS buffer | Provides detergent for unfolding and charge for electrophoresis |
| Reducing Agent Volume | Typically 1 µL of a 10X stock per 10 µL final volume [36] | Ensures sufficient molar excess to reduce all disulfide bonds | |
| 2. Heat Denaturation | Temperature | 70°C [36] | Adequately denatures most proteins while minimizing aggregation and degradation |
| Time | 2–10 minutes [36] | Balances complete unfolding with sample integrity | |
| 3. Post-Processing | Cooling | Brief centrifugation to collect condensation | Prevents uneven loading into the gel well |
Detailed Experimental Protocol: Sample Preparation for Reducing SDS-PAGE
The following protocol is designed for preparing reduced and denatured samples from a purified protein solution or a clarified cell lysate.
Materials and Reagents
Table 3: The Scientist's Toolkit - Essential Reagents for Sample Preparation
| Item | Function | Example Product/Catalog Number |
|---|---|---|
| SDS/LDS Sample Buffer (4X) | Denatures proteins, provides charge and dye for tracking | LDS Sample Buffer, 4X (e.g., NP0007) [36] |
| Reducing Agent (10X) | Cleaves disulfide bonds | NuPAGE Sample Reducing Agent (10X) (e.g., NP0004) [36] or 1M DTT / 0.5M TCEP |
| Cell Lysis Buffer | Extracts proteins from cells/tissues | RIPA Lysis Buffer or M-PER [36] |
| Protease Inhibitor Cocktail | Prevents protein degradation during extraction | Halt Protease and Phosphatase Inhibitor Cocktail (e.g., 78440) [36] |
| BCA Protein Assay Kit | Quantifies protein concentration for equal loading | Pierce BCA Protein Assay (e.g., 23225) [36] |
Step-by-Step Procedure
Prepare the Working Sample Mixture: In a microcentrifuge tube, combine the following reagents to a final volume of 10 µL:
- x µL Protein sample (e.g., 2 µL of a 5 µg/µL lysate for a 10 µg load)
- 2.5 µL 4X LDS/SDS Sample Buffer
- 1 µL 10X Reducing Agent (DTT or TCEP)
- y µL Deionized water to bring the total volume to 10 µL [36]
Denature and Reduce:
- Cap the tubes tightly and vortex briefly to mix.
- Heat the samples in a preheated heat block or water bath at 70°C for 10 minutes [36].
Prepare for Loading:
- Briefly centrifuge the samples (10-15 seconds) to bring down all condensation and liquid from the tube walls.
- The samples are now ready to be loaded onto a polyacrylamide gel.
Workflow and Decision Pathway
The following diagram summarizes the logical process for preparing protein samples for denaturing gel electrophoresis, incorporating key decision points for reduction and heating.
Accurate protein quantification is a critical prerequisite for successful denaturing gel electrophoresis, directly influencing loading precision, resolution, and interpretability of results. The Bradford and bicinchoninic acid (BCA) assays represent two predominant colorimetric methods for determining protein concentration, each with distinct chemistries and compatibility profiles. Selection between these assays is not trivial and must be informed by sample composition, presence of interfering substances, and required sensitivity. This application note provides a structured comparison and detailed protocols for both methods, specifically contextualized within sample preparation workflows for denaturing protein gel electrophoresis research.
Core Principles and Comparative Analysis
Fundamental Chemical Mechanisms
Bradford Assay: This method relies on the binding of Coomassie Brilliant Blue G-250 dye to proteins under acidic conditions. The dye undergoes a metachromatic shift from a reddish-brown form (absorption maximum at 465 nm) to a blue form (absorption maximum at 595 nm) upon primarily interacting with basic amino acid residues—especially arginine, lysine, and histidine—in proteins [45] [46]. The stabilized anionic blue form exhibits an absorbance peak at 595 nm, the intensity of which is proportional to protein concentration.
BCA Assay: The BCA method is a two-step reaction based on the biuret reaction and subsequent colorimetric detection. First, peptide bonds in the protein reduce cupric ions (Cu²⁺) to cuprous ions (Cu⁺) in an alkaline environment. Second, two molecules of bicinchoninic acid (BCA) chelate each Cu⁺ ion, forming a purple-colored complex that strongly absorbs light at 562 nm [47] [48]. The reduction reaction is also enhanced by specific amino acids: cysteine, cystine, tyrosine, and tryptophan [48].
The following diagram illustrates the core chemical principles and workflow logic for each assay.
Decision Framework: Bradford vs. BCA
The choice between Bradford and BCA assays is multifaceted. The following table summarizes key comparative parameters to guide researchers in selecting the appropriate assay for their specific application in preparing samples for denaturing gel electrophoresis.
Table 1: Comprehensive Comparison of Bradford and BCA Protein Assays
| Parameter | Bradford Assay | BCA Assay |
|---|---|---|
| Fundamental Principle | Dye-binding (Coomassie Blue), shift to 595 nm [47] [45] | Copper reduction & BCA chelation, absorption at 562 nm [47] [48] |
| Key Chemical Reactants | Basic residues (Arg, Lys, His) [45] [46] | Peptide bonds, Cys, Tyr, Trp residues [48] |
| Sensitivity Range | 1-20 µg/mL [49] | 25-2000 µg/mL (standard) [49] |
| Dynamic Range | Narrower [49] | Broader [49] |
| Assay Time | Quick (~5-10 minutes) [49] | Longer (30 min at 37°C to 2 hours) [47] [49] |
| Protein-to-Protein Uniformity | High variability; sensitive to basic amino acid content [47] [46] | More consistent across different protein types [47] [49] |
| Compatibility with Detergents | Low tolerance; detergents like SDS cause significant interference [49] [45] | High tolerance; compatible with ionic and non-ionic detergents [47] [48] |
| Compatibility with Reducing Agents | Generally compatible (DTT, β-mercaptoethanol) [50] | Sensitive to interference [51] [46] |
| Ideal Use Cases | Quick screening of pure proteins, limited sample volume, educational labs [49] | Samples with detergents (cell lysates), requiring consistency across different proteins [47] [49] |
Experimental Protocols
Reagent Solutions and Materials
Table 2: Essential Research Reagent Solutions
| Item | Function/Description | Example/Note |
|---|---|---|
| Bradford Reagent | Coomassie Brilliant Blue G-250 dye in methanol/phosphoric acid. Binds proteins for detection [52] [45]. | Prepare fresh or use commercial kit. Store in a brown bottle [52]. |
| BCA Working Reagent | A mixture of BCA Reagent A (containing Cu²⁺) and Reagent B (containing BCA) [51]. | Mix 50 parts A to 1 part B. Stable for one week [51]. |
| Protein Standard (BSA) | A known-concentration protein for generating a standard curve. | Bovine Serum Albumin (BSA) is most common. Prepare serial dilutions [52] [51]. |
| Homogenization/Dilution Buffer | A compatible buffer to dilute samples and standards without causing interference. | Tris-HCl or PBS are often suitable. Avoid high concentrations of interfering substances [51] [45]. |
| Spectrophotometer/Plate Reader | Instrument to measure the absorbance of the colored reaction product. | Must be capable of reading at 595 nm (Bradford) or 562 nm (BCA) [52] [51]. |
Step-by-Step Protocol: Bradford Assay
This protocol is adapted for a cuvette-based format but can be scaled to a microplate format [52] [45].
Workflow Overview:
Procedure:
Preparation of BSA Standard Curve:
Sample Preparation:
- Dilute the unknown protein sample to an estimated concentration within the linear range of the standard curve (e.g., 1:50 dilution in PBS) [45].
Reaction Setup:
- Pipette 20 µL of each standard and unknown sample into separate cuvettes.
- Add 1 mL of Bradford reagent to each cuvette and mix thoroughly by inversion [52].
Incubation and Measurement:
Data Analysis:
- Plot the absorbance values of the standards against their known concentrations to generate a standard curve.
- Perform linear regression analysis. The equation of the line (y = mx + c) is used to calculate the concentration of the unknown (x) based on its absorbance (y) [45].
Step-by-Step Protocol: BCA Assay
This protocol is based on the Pierce BCA method and is suitable for a microplate format [51].
Workflow Overview:
Procedure:
Preparation of BSA Standard Curve:
- Prepare a stock BSA solution (e.g., 2 mg/mL). Create a standard dilution series in a compatible buffer (e.g., 0, 0.125, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0 mg/mL) as per your experimental design [51].
Sample and Reagent Preparation:
Reaction Setup:
- Pipette 25 µL of each standard and unknown sample into the wells of a 96-well microplate, in duplicate.
- Add 200 µL of the BCA Working Reagent to each well. Mix the plate gently on a shaker [51].
Incubation and Measurement:
Data Analysis:
- Generate a standard curve by plotting the average absorbance of each standard against its concentration.
- Use the linear regression equation from the standard curve to calculate the protein concentration of the unknown samples, applying the relevant dilution factor [51].
Selecting the appropriate protein quantification assay is a critical decision that underpins the reliability of subsequent denaturing gel electrophoresis. The Bradford assay offers a rapid, sensitive solution for high-throughput screening of relatively pure protein samples. In contrast, the BCA assay, with its greater tolerance for common detergents and more uniform response across diverse proteins, is better suited for complex mixtures like cell lysates. By aligning the strengths of each method with the specific characteristics of your protein samples, you ensure accurate quantification, optimal gel loading, and ultimately, robust and reproducible research outcomes.
Sample preparation is a critical foundation for successful denaturing protein gel electrophoresis. The integrity of experimental data is heavily dependent on the initial steps of protein extraction and preparation. Complex samples, including cell lysates, high-salt samples, and viscous materials, present unique challenges that can compromise resolution, lead to artifacts, and render results uninterpretable if not properly addressed. Within the broader thesis of sample preparation methodology for denaturing protein gel electrophoresis research, this application note provides detailed protocols and evidence-based strategies for handling these challenging sample types, ensuring that researchers can achieve reliable, reproducible, and high-quality protein separation.
Fundamental Challenges in Complex Sample Preparation
The journey from a biological sample to a resolved protein band on a gel is fraught with potential pitfalls. Three common challenges—viscosity from genomic DNA, interference from high salt concentrations, and the presence of disruptive agents—can significantly impact the outcome.
Genomic DNA in Cell Lysates: The release of genomic DNA during cell lysis dramatically increases sample viscosity. This high viscosity affects pipetting accuracy, impedes uniform protein migration into the gel well, and results in smeared or distorted band patterns [9]. Furthermore, cell lysates contain both soluble and insoluble fractions, and the nature of the insoluble material can itself alter protein migration and resolution [9].
High Salt Concentrations: Samples with high ionic strength, such as those eluted from certain purification columns or solubilized in specific buffers, exhibit increased conductivity. This can lead to uneven heating within the gel, altered protein migration speeds, and the appearance of gel artifacts, even in adjacent lanes containing samples with normal salt content [9].
Guanidine-HCl and Ionic Detergents: Similar to high salt, guanidine-HCl creates high ionic strength conditions. Moreover, guanidine precipitates in the presence of SDS, leading to various types of gel artifacts [9]. While RIPA buffer is effective for lysis, the presence of Triton X-100 can subsequently inhibit the blotting of proteins less than 40 kDa [9].
Table 1: Common Challenges and Their Impacts on Electrophoresis
| Challenge | Primary Cause | Impact on Gel Electrophoresis |
|---|---|---|
| High Viscosity | Genomic DNA in cell lysates [9] | Smearing, distorted bands, poor resolution, difficult sample loading [9] |
| High Salt Concentration | High ionic strength buffers [9] | Increased conductivity, uneven heating, wavy or distorted bands, gel artifacts in adjacent lanes [9] |
| Precipitating Agents | Guanidine-HCl in the presence of SDS [9] | Gel artifacts, protein precipitation [9] |
| Blotting Inhibition | Triton X-100 in RIPA buffer [9] | Poor transfer of low molecular weight proteins (<40 kDa) to membrane [9] |
Experimental Protocols for Sample Preparation
Cell Lysis and Viscosity Reduction
The following protocol is optimized for preparing soluble protein fractions from mammalian cell cultures while addressing the issue of viscosity.
Materials:
- Lysis Buffer: Choose based on protein localization (e.g., RIPA for membrane-bound/nuclear, NP-40 for cytoplasmic) [36].
- Protease and Phosphatase Inhibitor Cocktail (e.g., Halt or Pierce) [36].
- Ice-cold Phosphate-Buffered Saline (PBS)
- Microcentrifuge Tubes, pre-cooled
Procedure for Adherent Cells:
- Preparation: Place the cell culture dish on ice. Aspirate the culture medium and wash the cells gently with ice-cold PBS [36].
- Lysis: Aspirate the PBS and add ice-cold lysis buffer, supplemented with protease and phosphatase inhibitors immediately before use (~1 mL per 10⁷ cells or a 100 mm plate) [36]. Gently shake or swirl the dish for 5 minutes on ice.
- Collection: Scrape the cells and transfer the lysate to a pre-cooled microcentrifuge tube.
- Clarification: Centrifuge at ~14,000 x g for 15 minutes at 4°C to pellet cell debris and insoluble material [36].
- Shear Genomic DNA: To reduce viscosity, shear the genomic DNA in the supernatant by brief sonication (e.g., 5-10 seconds on a low setting) or by passing the lysate several times through a narrow-gauge needle (e.g., 27-gauge) [9].
- Separation: Transfer the clarified, low-viscosity supernatant to a new tube. Discard the pellet [36].
Procedure for Suspension Cells:
- Pellet Cells: Centrifuge the cell suspension at 2,500 x g for 10 minutes. Discard the supernatant [36].
- Wash: Resuspend the cell pellet in ice-cold PBS and re-pellet by centrifugation.
- Lysis: Add ice-cold lysis buffer with inhibitors to the pellet. Pipette up and down to resuspend and shake gently for 10 minutes on ice [36].
- Clarification and DNA Shearing: Centrifuge at ~14,000 x g for 15 minutes. Transfer the supernatant and shear genomic DNA as described in Step 5 for adherent cells [9] [36].
Addressing High Salt and Guanidine-HCl
For samples containing high concentrations of salts or guanidine-HCl, the following clean-up procedures are recommended.
Materials:
- Dialysis Tubing or Cassettes (appropriate molecular weight cutoff)
- Low-Salt Buffer (e.g., Tris-HCl, NH₄HCO₃)
- Precipitation Reagents (e.g., Acetone, TCA/Acetone, Methanol/Chloroform)
Dialysis Protocol:
- Prepare Dialysis Setup: Load the sample into dialysis tubing sealed at both ends.
- Dialyze: Submerge the tubing in a large volume (e.g., 500x sample volume) of low-salt buffer (e.g., 25 mM Tris-HCl) [9]. Stir gently at 4°C.
- Buffer Change: Change the dialysis buffer at least twice over 24 hours.
- Recovery: Recover the dialyzed sample from the tubing.
Precipitation and Resuspension Protocol:
- Precipitate Proteins: Add 4-5 volumes of ice-cold acetone (or use TCA/acetone) to the sample. Vortex and incubate at -20°C for at least 1 hour [53].
- Pellet Proteins: Centrifuge at >10,000 x g for 10 minutes at 4°C. A protein pellet should be visible.
- Wash: Carefully remove the supernatant. Wash the pellet with cold 80% acetone to remove residual salts and agents.
- Air Dry: Briefly air-dry the pellet (do not over-dry, as this can make resuspension difficult).
- Resuspend: Solubilize the protein pellet in a compatible, low-salt electrophoresis sample buffer [9].
Table 2: Comparison of Sample Clean-Up Methods
| Method | Principle | Recommended Use | Advantages | Disadvantages |
|---|---|---|---|---|
| Dialysis [9] | Diffusion of small molecules through a semi-permeable membrane | Samples with very high volume or when maintaining protein activity is critical | Gentle; no protein loss; suitable for large volumes | Time-consuming (hours to days); requires specialized equipment |
| Precipitation/ Resuspension [9] | Dehydration and salting-out of proteins | Small-volume samples; rapid cleanup; removal of guanidine-HCl | Rapid; effective for salt and guanidine removal; concentrates sample | Potential for incomplete resuspension; may lose very small or hydrophilic proteins |
The Scientist's Toolkit: Research Reagent Solutions
Successful handling of complex samples requires the use of specific reagents. The table below details essential materials and their functions.
Table 3: Essential Reagents for Preparing Complex Samples
| Reagent Category | Specific Examples | Function | Application Notes |
|---|---|---|---|
| Lysis Buffers | RIPA Buffer [36] [53], NP-40 Lysis Buffer [36] [53], M-PER [36] | Disrupts cell membranes to release proteins; choice depends on protein localization and necessity to preserve protein interactions. | RIPA: Whole cell, membrane, nuclear. NP-40: Cytoplasmic, mild. M-PER: Whole cell, non-denaturing. |
| Protease Inhibitors | PMSF (Serine proteases) [53], Aprotinin (Serine proteases) [53], EDTA (Metalloproteases) [53], Commercial Cocktails (e.g., Halt) [36] | Prevents protein degradation by inhibiting endogenous proteases released during lysis. | Add to lysis buffer immediately before use. Use cocktails for broad-spectrum protection. |
| Phosphatase Inhibitors | Sodium Orthovanadate [53], Sodium Fluoride [53], β-glycerophosphate [53] | Preserves protein phosphorylation states by inhibiting phosphatases. | Critical for detection of phospho-proteins. |
| Reducing Agents | Dithiothreitol (DTT), 50 mM [9], β-mercaptoethanol, 2.5% [9], Tris(2-carboxyethyl)phosphine (TCEP), 50 mM [9] | Breaks disulfide bonds to fully denature proteins for separation by molecular weight. | Add fresh before heating; avoid long-term storage of reduced samples due to reoxidation [9]. |
| Nuclease Enzymes | DNase I, Benzonase | Degrades genomic DNA to reduce sample viscosity without shearing. | An alternative to mechanical shearing. |
| Protein Assays | BCA Assay [36], Bradford Assay [36] | Quantifies protein concentration to ensure equal loading across gel lanes. | BCA is more compatible with samples containing up to 5% detergents [36]. |
Workflow for Complex Sample Preparation
The following diagram provides a logical workflow for processing complex samples, from initial assessment to final preparation for gel loading.
Critical Notes for Denaturing Electrophoresis
- Sample Heating: Avoid heating samples at 100°C in SDS-containing buffer, as this can promote proteolysis. Instead, heat samples at 85°C for 2–5 minutes for optimal results [9]. For native electrophoresis, do not heat the samples [9].
- Reducing Agents: Add reducing agents shortly before use (within an hour of loading the gel) and avoid long-term storage of reduced samples due to reoxidation, which produces inconsistent results [9]. If reduced and non-reduced samples must be run on the same gel, do not load them in adjacent lanes to prevent diffusion of reducing agent [9].
- Protein Quantification: Accurate quantification after sample clean-up is essential. The BCA assay is often preferred over the Bradford assay for samples that have undergone processing, as it is less affected by compositional differences and compatible with most lysis buffer components [36].
In denaturing protein gel electrophoresis research, the step of combining a protein sample with SDS-PAGE loading dye represents a critical transition from crude biological extract to analytically ready specimen. This process, which creates the "final loading mix," dictates the success of all subsequent separation, analysis, and interpretation phases. Proper execution ensures that proteins are uniformly denatured, linearized, and impartially prepared for molecular weight-based separation through the polyacrylamide matrix [5]. The loading dye mixture serves multiple essential functions: it provides the denaturing environment through sodium dodecyl sulfate (SDS), facilitates disulfide bond reduction when supplemented with appropriate agents, confers density for gel loading, and includes a visual tracking dye for monitoring electrophoresis progression [54]. Within the broader context of sample preparation methodology, this specific protocol establishes the foundation for reliable protein analysis across diverse applications including drug development, biomarker discovery, and quality control in biopharmaceutical manufacturing [5] [55].
Principles of Denaturing Sample Preparation
The fundamental objective of creating the final loading mix is to completely dismantle higher-order protein structures while imparting a uniform charge-to-mass ratio to all polypeptides. SDS, an anionic detergent, plays the central role in this process by binding to hydrophobic regions of proteins at a relatively constant ratio of approximately 1.4 grams of SDS per gram of protein [55]. This SDS coating masks the proteins' intrinsic charges, conferring a net negative charge that is proportional to polypeptide length. Simultaneously, the reducing agent component, typically dithiothreitol (DTT) or β-mercaptoethanol, targets disulfide bonds that stabilize tertiary and quaternary structures [9]. The effectiveness of this denaturation process directly impacts electrophoretic resolution, as incomplete denaturation results in aberrant migration patterns, smearing, and inaccurate molecular weight determination [56]. The entire process transforms complex three-dimensional protein structures into linear, negatively charged SDS-polypeptide complexes that migrate strictly according to molecular weight when subjected to an electric field within the polyacrylamide gel matrix [5].
Composition of SDS-PAGE Loading Dye
The loading dye formulation is a precisely balanced chemical system designed to address multiple technical requirements of the electrophoresis process. Each component serves a specific function that collectively ensures optimal protein separation and visualization.
Table 1: Core Components of SDS-PAGE Loading Dye
| Component | Standard Concentration | Primary Function | Technical Considerations |
|---|---|---|---|
| SDS | 1-2% (w/v) | Denatures proteins and confers negative charge | Ensures constant charge-to-mass ratio; critical for molecular weight-based separation [54] [55] |
| Reducing Agent | DTT: 50 mM; β-mercaptoethanol: 2.5% | Breaks disulfide bonds | DTT preferred for stronger reducing power; add fresh before use [9] |
| Glycerol | 10-20% (v/v) | Increases density for well loading | Precomes sample diffusion into running buffer [56] |
| Tracking Dye | Bromophenol blue: 0.01-0.02% | Visualizes migration front | Monitors electrophoresis progress without protein interference [56] |
| Buffer | 50-100 mM Tris-HCl, pH 6.8 | Maintains optimal pH environment | Stabilizes proteins during denaturation; compatible with gel buffer systems [56] |
Beyond these core components, specialized applications may require modifications to the standard formulation. For example, Tris(2-carboxyethyl)phosphine (TCEP) serves as an alternative reducing agent at 50 mM final concentration, offering advantages over conventional reducers through its superior stability and resistance to air oxidation [9]. For samples containing high salt concentrations, which increase conductivity and cause migration artifacts, the loading dye may be prepared at higher concentrations to effectively dilute these interfering substances during the final mixing step [9].
Recommended Protocols
Standard Sample Preparation Workflow
The following protocol describes the systematic process for creating the final loading mix from purified protein samples, ensuring consistent and reliable results for denaturing SDS-PAGE.
Table 2: Protocol for Preparing Final Loading Mix
| Step | Procedure | Parameters & Considerations |
|---|---|---|
| 1. Protein Quantification | Determine protein concentration using BCA or Bradford assay | Ensure equal loading across lanes (typically 10-50 μg per lane) [57] |
| 2. Sample Mixing | Combine protein sample with loading dye at recommended ratio (typically 1:1 to 1:4) | Volumes should yield 10-40 μL final volume per lane; adjust based on well capacity |
| 3. Denaturation | Heat mixture at 85°C for 2-5 minutes [9] | Avoid higher temperatures (e.g., 100°C) to prevent proteolysis [9] |
| 4. Brief Centrifugation | Pulse spin (10-15 seconds) to collect condensate | Eliminates air bubbles and ensures complete sample recovery |
| 5. Gel Loading | Immediately load onto polyacrylamide gel or store at -20°C | For stored samples, reheat briefly (70°C, 1-2 minutes) before loading |
The following workflow diagram illustrates the critical steps in preparing the final loading mix:
Specialized Methodological Considerations
Specific sample types and experimental requirements necessitate modifications to the standard protocol:
Cell Lysates: Samples containing genomic DNA require additional processing to reduce viscosity. Mechanical shearing through narrow-gauge needle passage or benchtop centrifugation is recommended before mixing with loading dye [9]. For lysates prepared with RIPA buffer, note that subsequent Western blotting of proteins less than 40 kDa may be inhibited due to Triton X-100 interference [9].
Reduced vs. Non-Reduced Conditions: For reduced SDS-PAGE, adding fresh reducing agent immediately before denaturation is critical. To prevent reoxidation during storage, avoid preparing reduced samples more than one hour before electrophoresis [9]. When both reduced and non-reduced samples must be analyzed on the same gel, ensure they are not loaded in adjacent lanes to prevent reducing agent diffusion from affecting non-reduced samples [9].
Problematic Samples: Samples containing high salt concentrations or solubilizing agents such as guanidine-HCl require special handling. High ionic strength increases conductivity, resulting in aberrant migration patterns and gel artifacts in adjacent lanes [9]. For such samples, implement buffer exchange through dialysis or precipitation/resuspension in low-salt buffer before mixing with loading dye.
Troubleshooting Common Issues
Despite the apparent simplicity of sample preparation, several technical challenges can compromise electrophoretic results. The following table addresses common problems and their solutions:
Table 3: Troubleshooting Guide for Final Loading Mix Preparation
| Problem | Potential Causes | Recommended Solutions |
|---|---|---|
| Smearing or distorted bands | Sample overloading [57]; incomplete denaturation [56]; high salt concentration [9] | Reduce protein amount (10-50 μg/lane) [57]; ensure proper heating [56]; desalt samples [9] |
| Poor band resolution | Incorrect acrylamide percentage; expired reagents; improper buffer conditions | Match gel percentage to protein size; use fresh loading dye; ensure correct pH [56] |
| Vertical streaking | Protein precipitation; insufficient SDS; trapped air bubbles | Increase SDS concentration; degas solutions; centrifuge before loading [56] |
| Faint or invisible bands | Insufficient protein [57]; protein degradation; improper staining | Confirm concentration quantification [57]; add protease inhibitors; verify staining protocol [56] |
| Artifact bands | Protein aggregation; proteolysis; reoxidation of reduced samples | Fresh reducing agents; work on ice; add urea for solubilization [9] [56] |
Research Reagent Solutions
Successful execution of the final loading mix protocol requires specific high-quality reagents optimized for protein electrophoresis. The following essential materials represent the core toolkit for researchers:
Table 4: Essential Research Reagent Solutions
| Reagent | Function | Application Notes |
|---|---|---|
| SDS Solution (10-20%) | Denatures proteins and confers charge | Use electrophoretic-grade SDS for consistent binding [54] |
| Dithiothreitol (DTT, 1M stock) | Reduces disulfide bonds | Preferred over β-mercaptoethanol for lower odor and stronger reduction [9] |
| Tris(2-carboxyethyl)phosphine (TCEP) | Alternative reducing agent | More stable than DTT; resistant to air oxidation [9] |
| Protease Inhibitor Cocktails | Prevents protein degradation | Essential for cell lysates; add to lysis buffer [32] |
| Protein Quantification Kits (BCA/Bradford) | Measures protein concentration | BCA preferred for compatibility with detergents [32] |
| Commercial Loading Dye Formulations | Ready-to-use mixtures | Provide consistency; available with various reducing agents [56] |
The preparation of the final loading mix represents a critical juncture in denaturing protein gel electrophoresis methodology, where biochemical precision directly determines analytical outcomes. Through systematic application of the protocols detailed in this document—including proper denaturation conditions, appropriate reducing agent selection, and specialized handling for challenging samples—researchers can ensure the integrity of their electrophoretic separations. The troubleshooting guidelines and reagent specifications provide a practical framework for addressing common technical challenges, while the foundational principles illuminate the scientific rationale behind each procedural step. When executed with meticulous attention to detail, this essential sample preparation step transforms complex protein mixtures into analytically tractable specimens, enabling accurate molecular weight determination, purity assessment, and functional characterization across diverse research applications.
Troubleshooting SDS-PAGE: Solving Smearing, Distortion, and Poor Resolution
Within the critical context of sample preparation for denaturing protein gel electrophoresis research, protein smearing is a common yet debilitating artifact that can obfuscate results and compromise data interpretation. For researchers, scientists, and drug development professionals, distinguishing the root cause of smearing is the essential first step toward its resolution. This application note focuses on two primary culprits: protein degradation and improper denaturation. We provide a detailed guide to diagnose the origin of smearing in your experiments and outline robust protocols to rectify these issues, ensuring the integrity of your electrophoretic data.
Differential Diagnosis: Degradation vs. Improper Denaturation
Accurately diagnosing the cause of smearing is paramount. The following table contrasts the characteristic gel appearances and key indicators for degradation versus improper denaturation.
Table 1: Diagnostic Features to Distinguish Protein Degradation from Improper Denaturation
| Diagnostic Feature | Protein Degradation | Improper Denaturation |
|---|---|---|
| Primary Cause | Proteolytic activity or chemical damage [58] | Incomplete unfolding or disruption of protein structure [59] [60] |
| Typical Gel Appearance | A "ladder" of multiple lower molecular weight bands below the expected protein size; random fuzzy background [58] | A continuous, dense smear extending from the top of the gel; persistent high molecular weight aggregates [61] [62] |
| Key Diagnostic Test | Compare samples heated immediately vs. left at room temperature before heating [58] | Vary the heating temperature and duration, or change denaturant/reducing agent conditions [9] |
| Effect of Protease Inhibitors | Smearing is reduced or eliminated [58] | No effect on smearing |
| Common Underlying Issues | - Protease contamination- Delayed heating after lysate preparation- Asp-Pro bond cleavage from excessive heat [58] | - Insufficient heating temperature/duration- Inadequate or oxidized reducing agents- Incorrect sample buffer composition [9] |
The following workflow provides a logical sequence for diagnosing and correcting the source of protein smearing in SDS-PAGE.
In-Depth Analysis and Resolution Protocols
Protocol A: Resolving Protein Degradation
Protein degradation occurs when proteases present in the sample cleave the protein of interest before or during sample preparation, leading to a heterogeneous mixture of fragments [58].
Detailed Experimental Protocol
Sample Preparation with Inhibitors:
- Prepare fresh lysis buffer supplemented with a broad-spectrum protease inhibitor cocktail.
- Ensure the buffer is chilled on ice.
- Immediately after lysing cells or tissues, mix the lysate thoroughly with the prepared SDS-PAGE sample buffer.
Controlled Heating:
- Do not leave samples in SDS buffer at room temperature without heating.
- Heat samples at 75–85°C for 2–5 minutes [58] [9]. Heating at 100°C can cause cleavage at Asp-Pro bonds, while 75°C is sufficient to inactivate most proteases without causing this specific damage [58].
- After heating, briefly centrifuge samples (e.g., 17,000 x g for 2 minutes) to pellet any insoluble material before loading the gel [58].
Diagnostic Test for Proteolysis:
- Split your sample into two aliquots after adding sample buffer.
- Heat one aliquot immediately as described above.
- Leave the other aliquot at room temperature for 2–4 hours, then heat it.
- Run both samples on the same gel. Increased smearing or the appearance of lower molecular weight bands in the room-temperature sample confirms proteolytic degradation [58].
Protocol B: Resolving Improper Denaturation
Improper denaturation results from the failure to completely unfold the protein and coat it with SDS, which prevents it from migrating according to its true molecular weight. This often manifests as a high molecular weight smear or aggregation at the top of the gel [61] [62].
Detailed Experimental Protocol
Optimize Denaturation and Reduction:
- Heating: Use the recommended 85°C for 2–5 minutes for optimal denaturation without promoting proteolysis or specific bond cleavage [9].
- Reducing Agents: Use fresh reducing agents to break disulfide bonds.
Verify Sample Buffer Composition:
- Ensure the sample buffer contains an adequate concentration of SDS. A general guideline is a 3:1 mass ratio of SDS to protein [58].
- For difficult samples (e.g., membrane proteins), consider adding 6–8 M urea or a non-ionic detergent like Triton X-100 to the sample buffer to aid solubilization [58].
- Avoid high salt concentrations (>100 mM) in your sample, as they increase conductivity and can cause smearing and distorted bands. Dialyze, precipitate, or dilute samples into a low-salt buffer if necessary [63] [9].
Diagnostic Test for Incomplete Denaturation:
- Test different heating conditions (e.g., 70°C vs. 85°C vs. 95°C) for a fixed time.
- Compare samples with and without a fresh, potent reducing agent (like TCEP).
- A reduction in smearing with more stringent denaturation conditions confirms improper denaturation as the likely cause.
The Scientist's Toolkit: Essential Research Reagents
The following table lists key reagents critical for preventing protein smearing, along with their specific functions and recommended usage.
Table 2: Key Research Reagents for Preventing Protein Smearing
| Reagent | Function | Application Notes |
|---|---|---|
| Protease Inhibitor Cocktail | Inhibits a wide spectrum of serine, cysteine, metallo-, and acid proteases, preventing protein degradation [58]. | Add fresh to lysis buffer immediately before use. |
| Dithiothreitol (DTT) | Reducing agent that breaks disulfide bonds within and between proteins, aiding complete unfolding [9]. | Use at 50 mM final concentration. Less stable than TCEP over time. |
| Tris(2-carboxyethyl)phosphine (TCEP) | Reducing agent that breaks disulfide bonds. More stable and resistant to oxidation than DTT [9]. | Use at 50 mM final concentration. Preferred for long-term storage of reducing buffers. |
| Sodium Dodecyl Sulfate (SDS) | Ionic detergent that denatures proteins and confers a uniform negative charge, essential for separation by size [58]. | Maintain a 3:1 mass ratio of SDS to protein to ensure complete coating [58]. |
| Urea | Chaotropic agent that disrupts hydrogen bonds, aiding in the solubilization and denaturation of difficult proteins [58]. | Use at 6-8 M concentration for membrane proteins or aggregates. Treat solutions with mixed-bed resin to remove cyanate ions that cause carbamylation [58]. |
Protein smearing in SDS-PAGE is a solvable problem. A systematic approach to diagnosis, leveraging the distinct gel patterns and targeted diagnostic tests outlined herein, allows researchers to efficiently pinpoint whether degradation or improper denaturation is at fault. By implementing the corresponding detailed protocols—emphasizing stringent protease inhibition, optimized heating, and fresh, effective reducing agents—scientists can eliminate smearing artifacts. This ensures the generation of clean, reliable, and interpretable data, thereby upholding the rigorous standards required for successful research and drug development.
Eliminating 'Smeling' and 'Frowning' Bands Caused by Uneven Heat Dissipation
In denaturing protein gel electrophoresis, the integrity of data hinges on the quality of the separation, which can be severely compromised by band distortions known as 'smiling' and 'frowning.' These artifacts are symptomatic of uneven heat dissipation across the gel, a prevalent issue that can obscure results and lead to erroneous conclusions in drug development research. This application note, framed within the critical context of sample preparation for protein research, delineates the underlying causes of these distortions and provides detailed, actionable protocols to eliminate them, thereby ensuring the reproducibility and reliability of electrophoretic data.
‘Smiling’ and ‘frowning’ bands are visual artifacts where bands curve upwards or downwards, respectively, instead of migrating in straight lines. These distortions are not merely cosmetic; they indicate non-uniform electrophoretic conditions that can compromise the accuracy of molecular weight determination, quantitation, and the clear resolution of protein species.
The primary culprit behind these phenomena is Joule heating—heat generated as current passes through the resistive gel matrix. If this heat is not dissipated evenly, a temperature gradient develops across the gel. In a typical horizontal setup, the center of the gel is often warmer than the edges. This warmer center reduces the viscosity of the buffer and gel, allowing proteins in the middle lanes to migrate faster, resulting in upward-curving 'smiling' bands [64]. Conversely, ‘frowning’ bands, where the edges migrate faster, can occur if the edges are warmer, such as in a poorly configured vertical gel apparatus [65].
For researchers relying on SDS-PAGE for analyzing protein purity, complex formation, or expression levels, these distortions can cause bands from adjacent lanes to overlap, making analysis unreliable. Therefore, controlling heat distribution is a fundamental aspect of high-quality sample preparation and electrophoretic separation.
Fundamental Causes and Preventive Strategies
A systematic approach to preventing band distortions involves addressing several key factors, from equipment setup to sample composition.
Electrophoresis Conditions and Hardware
The management of Joule heating is paramount. The following strategies are critical:
- Voltage and Current Control: Running the gel at an excessively high voltage generates intense heat. Implementing a constant current mode on the power supply, rather than constant voltage, helps maintain a more uniform rate of heat generation throughout the run [64]. If distortions persist, the first corrective action should be to reduce the applied voltage and extend the run time accordingly.
- Apparatus Setup: An improperly seated gel, crooked electrodes, or uneven buffer levels can create a non-uniform electric field, exacerbating local heating and migration anomalies [64]. Always verify that the gel is level and that buffer levels are equal across the tank.
- Buffer Composition and Volume: The use of fresh buffer at the correct concentration is essential. Depleted or incorrect buffer can alter the system's resistance and ion mobility, leading to inconsistent heating [63] [64]. Ensure an adequate buffer volume to act as a heat sink.
Sample-Related Factors
The composition of the sample itself can be a significant source of local heating and distortion.
- Salt Concentration: Samples containing high concentrations of salts create zones of high conductivity (low resistance) within the wells. This leads to localized heating and distortion of the electric field, which can pull bands into a smile or frown [63] [64].
- Sample Load: Overloading a well can overwhelm the local buffer capacity and produce a similar high-conductivity effect, leading to distorted bands [64].
Table 1: Summary of Causes and Preventive Strategies for Band Distortions
| Category | Specific Cause | Preventive Strategy |
|---|---|---|
| Electrophoresis Conditions | Excessive Voltage | Use constant current mode; reduce voltage and extend run time [64]. |
| Uneven Electric Field | Ensure gel is level, electrodes are straight, and buffer levels are even [64]. | |
| Depleted/Incorrect Buffer | Always use fresh buffer at the recommended concentration and ionic strength [64]. | |
| Sample Properties | High Salt Concentration | Desalt samples via dialysis or precipitation; dilute in nuclease-free water [63]. |
| Overloading | Load a smaller volume or a more diluted sample to avoid exceeding well capacity [64]. |
The following workflow outlines a systematic protocol for diagnosing and correcting smiling and frowning effects.
Detailed Experimental Protocols
The following protocols provide a step-by-step guide to executing the key strategies for preventing uneven heat dissipation.
Protocol: Optimizing Electrophoresis Run Conditions for Minimal Distortion
This protocol is designed for standard SDS-PAGE using a vertical gel apparatus.
I. Materials and Reagents
- Pre-cast or freshly cast polyacrylamide gel
- SDS-PAGE running buffer (e.g., 1x Tris-Glycine-SDS)
- Constant current power supply
- Pre-stained protein molecular weight standard
II. Procedure
- Assemble the Gel Apparatus: Ensure the gel cassette is properly seated in the tank according to the manufacturer's instructions. Fill the inner and outer chambers with fresh running buffer, checking for leaks.
- Load Samples and Marker: Load an appropriate volume of pre-stained protein ladder and your experimental samples into the wells. The pre-stained marker will allow real-time monitoring of the run.
- Set Power Supply Parameters:
- Initial Settings: Begin the run at a constant current of 10-15 mA per gel (for a mini-gel system). This low initial current minimizes heat generation as the samples stack in the stacking gel.
- Main Separation: Once the samples have entered the resolving gel (after approximately 15-30 minutes), the current can be increased. Do not exceed 25-30 mA per gel. For multiple gels, adjust the total current accordingly.
- Voltage Limiting: As a safety precaution, set a voltage limit (e.g., 200V) to prevent a runaway increase in voltage, which can occur as the ion fronts move through the gel.
- Monitor the Run: If possible, run the electrophoresis in a cold room (4°C) or use a built-in cooling apparatus. Observe the migration of the pre-stained marker. If smiling begins to appear, temporarily lower the current.
- Completion: Stop the run when the dye front has reached the bottom of the gel. Proceed with fixation and staining.
Protocol: Sample Preparation and Desalting
This protocol describes a simple dialysis procedure to reduce salt content in protein samples.
I. Materials and Reagents
- Dialysis tubing or cassettes with appropriate molecular weight cut-off (MWCO)
- Dialysis buffer (e.g., 50 mM Tris-HCl, pH 8.0)
- Magnetic stirrer and stir bar
- Refrigerated cabinet (4°C)
II. Procedure
- Prepare Dialysis Tubing: If using tubing, cut an appropriate length and pre-treat it according to the manufacturer's instructions (e.g., boiling in EDTA, rinsing thoroughly).
- Load the Sample: Close one end of the tubing with a clip. Pipette the protein sample into the tubing, leaving space for expansion. Seal the top end with another clip, ensuring no leaks.
- Dialyze: Submerge the sealed dialysis bag in a large volume of dialysis buffer (a sample-to-buffer ratio of 1:1000 is recommended). Stir gently at 4°C for 4-16 hours.
- Change Buffer: Replace the dialysis buffer with a fresh volume and continue dialysis for another 4-16 hours.
- Recover Sample: Carefully remove the dialyzed sample from the tubing. The sample is now ready to be mixed with SDS-PAGE loading buffer.
Table 2: Troubleshooting Guide for Smiling and Frowning Bands
| Observed Problem | Potential Cause | Solution | Key Parameter to Check |
|---|---|---|---|
| Severe 'Smiling' | Excessive Joule heating in gel center. | Lower the running current/voltage; use a cooling device [64]. | Running current (mA). |
| 'Frowning' Bands | Edges warmer than center; poor gel contact. | Check gel seating and buffer levels; ensure electrodes are straight [64]. | Buffer level uniformity. |
| Smiling in One Lane | High salt concentration in a specific sample. | Desalt the offending sample via dialysis or spin colum [63] [64]. | Sample conductivity. |
| Persistent Distortion | Inefficient buffer ion mobility or depleted buffer. | Replace with freshly prepared running buffer [64]. | Buffer age and pH. |
The Scientist's Toolkit: Essential Reagents and Materials
The following table details key reagents and materials crucial for preventing heat-related distortions in denaturing protein gel electrophoresis.
Table 3: Research Reagent Solutions for Optimal Electrophoresis
| Item | Function and Importance | Optimal Use Note |
|---|---|---|
| Constant Current Power Supply | Provides stable, controlled electrical input to minimize erratic heat generation; essential for reproducible runs [64]. | Prefer models with programmable methods (e.g., step-wise current control) and voltage/current limiting features. |
| Tris-Glycine-SDS Running Buffer | Maintains stable pH and provides ions for conductivity. Fresh buffer ensures consistent resistance and heat profile [64]. | Prepare fresh or use aliquots from a sterile stock. Avoid more than 2-3 reuses to prevent ion depletion. |
| Pre-cast Polyacrylamide Gels | Offer superior consistency in gel polymerization and thickness, leading to more uniform electrical resistance and heat distribution. | Ensure the gel percentage is appropriate for your target protein size range. Store as recommended. |
| Dialysis Tubing/Cassettes | Critical for removing high concentrations of salts, detergents, or other small molecules from protein samples prior to loading [63]. | Select a MWCO that is 2-3 times smaller than the molecular weight of your target protein to prevent loss. |
| β-Mercaptoethanol or DTT | Strong reducing agents that break disulfide bonds, ensuring complete protein denaturation and preventing smearing that can complicate distortion analysis [65]. | Always add fresh to the loading buffer just before use, as it can oxidize over time. |
| High-Purity SDS | Anionic detergent that binds to and denatures proteins, imparting a uniform negative charge-to-mass ratio, which is the basis of SDS-PAGE separation [65]. | Use electrophoresis-grade SDS to ensure purity and consistent results. |
Within the framework of thesis research on sample preparation for denaturing protein gel electrophoresis, achieving high band resolution is a fundamental prerequisite for obtaining reliable and reproducible data. Poor band resolution, characterized by diffuse, overlapping bands, compromises the accuracy of molecular weight determination, quantitation, and subsequent analyses such as western blotting. This application note details a systematic, evidence-based protocol for optimizing three critical experimental parameters—gel concentration, applied voltage, and run time—to resolve protein bands with high clarity and precision, ensuring the integrity of data for drug development and research.
The root of poor resolution often lies in an imbalance between the sieving properties of the gel matrix and the electrophoretic conditions [64]. An incorrect gel pore size fails to adequately separate proteins by molecular weight, while suboptimal voltage and run time can lead to band broadening due to diffusion or Joule heating [66] [67]. The following sections provide quantitative guidance and detailed methodologies to diagnose and correct these issues, transforming a critical laboratory technique from a source of frustration into a robust and predictable tool.
The Scientist's Toolkit: Essential Reagents and Materials
The following table catalogues the essential materials required for the optimization experiments described in this protocol.
Table 1: Key Research Reagent Solutions for Gel Electrophoresis Optimization
| Item | Function/Description |
|---|---|
| Acrylamide/Bis-acrylamide | Forms the polyacrylamide gel matrix; the ratio and concentration determine pore size for molecular sieving [68]. |
| SDS-PAGE Sample Buffer (Laemmli Buffer) | Denatures proteins and confers a uniform negative charge, allowing separation primarily by molecular weight. |
| Tris-Glycine-SDS (TGS) Running Buffer | Maintains stable pH and conductivity during electrophoresis, ensuring consistent protein migration [69]. |
| Pre-stained Protein Ladder | Provides molecular weight standards for real-time monitoring of electrophoresis progress and post-run size estimation. |
| Pre-cast Gels or Gel Casting Apparatus | Pre-cast gels offer convenience and reproducibility; a casting apparatus is required for hand-poured gels [68]. |
| Vertical Electrophoresis System & Power Supply | The chamber holds the gel and buffer; the power supply delivers a controlled electrical field (constant current/voltage/power) [67]. |
Parameter Optimization: A Quantitative Guide
Optimal separation is achieved when the gel's pore size is matched to the size range of the target proteins, and the electrical conditions are tuned to minimize heat-induced band distortion while allowing sufficient time for separation.
Gel Concentration Optimization
The gel concentration is the most critical factor for resolution, as it dictates the sieving properties of the matrix [64]. The table below provides recommended polyacrylamide concentrations for separating proteins of different size ranges.
Table 2: Optimizing Gel Concentration for Protein Size Range
| Target Protein Size (kDa) | Recommended Gel Concentration (% Acrylamide) | Expected Outcome |
|---|---|---|
| 5 - 50 | 12% - 15% | High resolution for low molecular weight proteins; tighter pore size. |
| 30 - 100 | 10% - 12% | Standard range for most common analytical applications. |
| 50 - 200 | 8% - 10% | Better separation and migration for higher molecular weight proteins. |
| > 150 | 4% - 8% (Stacking Gel: 4%) | Large pore size allows very large proteins to enter and migrate. |
Voltage and Run Time Optimization
The applied voltage and run time are intrinsically linked. Higher voltages shorten run times but generate more Joule heat, which can cause band smiling, smearing, and diffusion [67] [64]. The relationship between band dispersion ((w)), electric field strength ((E)), and runtime ((t)) can be described by polynomial approximations that incorporate the effects of Joule heating [66].
Table 3: Optimizing Voltage and Run Time Conditions
| Goal | Recommended Voltage | Recommended Run Time | Rationale |
|---|---|---|---|
| Highest Resolution | Lower voltage (e.g., 80-100 V) | Longer time (e.g., 1.5-2 hours) | Minimizes heat generation, reducing band broadening and smiling [64]. |
| Fast Results | Higher voltage (e.g., 150-200 V) | Shorter time (e.g., 45-60 min) | Increases migration rate at the cost of potential heat-related artifacts. |
| Standard Analytical Run | Constant 120-150 V | Until dye front reaches bottom | A common balance between speed and resolution for routine analysis. |
Optimization Workflow
Detailed Experimental Protocols
Protocol 1: Systematic Gel Concentration Screening
This protocol is designed to empirically determine the optimal gel concentration for a target protein.
- Gel Preparation: Prepare or obtain 4-5 mini-gels with acrylamide concentrations spanning the expected optimal range (e.g., 8%, 10%, 12%, and 15%).
- Sample Preparation: Dilute the protein sample to a standard concentration (e.g., 1 µg/µL) in 1X SDS-PAGE sample buffer. Denature at 95°C for 5 minutes.
- Loading and Electrophoresis: Load an equal mass (e.g., 20 µg) of the prepared sample into each gel. Include a pre-stained protein ladder in one well. Run all gels simultaneously under identical conditions (e.g., constant 120 V) in the same or equivalent tanks until the dye front approaches the bottom.
- Analysis: Visualize the proteins using a preferred staining method (e.g., Coomassie Blue, silver stain, or in-gel fluorescence for tagged proteins [69]). Identify the gel concentration that yields the sharpest, most well-separated bands for the protein(s) of interest.
Protocol 2: Fine-Tuning Voltage and Run Time
Once the gel concentration is optimized, this protocol fine-tunes the electrophoretic conditions.
- Baseline Setup: Use the optimal gel concentration from Protocol 1. Prepare identical samples.
- Variable Application: Run multiple identical gels, each at a different constant voltage. Suggested test points: 80 V, 120 V, 160 V, and 200 V.
- Monitoring and Termination: For each run, note the time when the dye front reaches the bottom of the gel.
- Resolution Assessment: After staining, compare the band sharpness and separation across the different conditions. Lower voltages will typically produce sharper bands but require longer run times [67] [64]. The ideal condition is the highest voltage that does not produce visible smiling, smearing, or loss of resolution.
The interplay of electric field strength (E) and runtime (t) is critical. An E-t band model can be used to predict band dispersion, where bandwidth squared ((w^2)) is proportional to the product of runtime and an effective temperature, which itself is a function of the applied electric field [66]. This relationship underscores why longer runs at lower voltage often improve resolution by mitigating Joule heating effects.
Advanced Applications and Visualization
For researchers employing fluorescent protein (FP) fusions, a powerful application is the direct detection of in-gel fluorescence (IGF). This method bypasses the need for protein transfer and immunoblotting, providing clearer data with less background and a broader dynamic range [69].
Protocol: In-Gel Fluorescence Detection for FP-Tagged Proteins
- Sample Preparation: Prepare cell extracts expressing the FP-tagged protein of interest. Mix the extract with standard SDS-PAGE sample buffer. A critical deviation from standard protocol is to omit heating or to heat at a lower temperature (e.g., 42-55°C for 5-10 min) to partially preserve fluorescence, though some robust FPs (e.g., sfGFP) can withstand 95°C [69].
- Electrophoresis: Load and run the samples on a gel using the optimized concentration and voltage conditions. It is advisable to run the gel in the dark to prevent photobleaching.
- Direct Imaging: Immediately after electrophoresis, place the gel on a standard fluorescence gel imager. Use the appropriate excitation/emission settings for the specific FP (e.g., 488/530 nm for GFP).
- Post-Analysis: Following fluorescence imaging, the same gel can be subjected to total protein staining (e.g., Coomassie) to assess total protein load and profile.
IGF Workflow
Addressing the 'Edge Effect' and Other Lane-Specific Artifacts
In the realm of denaturing protein gel electrophoresis, the integrity of data is paramount for accurate analysis in research and drug development. A persistent challenge that compromises this integrity is the occurrence of lane-specific artifacts, most notably the 'edge effect'. This phenomenon, where samples in the outermost lanes of a gel exhibit distorted migration patterns compared to those in the center, poses a significant threat to the reproducibility and reliability of experimental results [70]. Within the broader thesis of optimal sample preparation, it is critical to understand that even perfectly prepared samples can yield aberrant data if electrophoretic conditions are suboptimal. This application note delineates the root causes of the edge effect and other common lane-specific artifacts and provides detailed, actionable protocols for their mitigation, ensuring that data quality is maintained across every lane of the gel.
Understanding the Artifacts: Causes and Consequences
The Edge Effect
The edge effect is visually characterized by distorted, curved, or smeared bands in the outermost lanes of a polyacrylamide gel, particularly when the peripheral wells are left empty [70]. The primary cause is a non-uniform electric field across the gel. The electric field strength is higher at the edges of the gel than in the center, a phenomenon exacerbated by the physical configuration of the gel cassette and buffer chambers [71]. This gradient leads to increased local heating and faster migration of samples in the edge lanes, resulting in the characteristic distortion that compromises lane-to-lane comparability.
Other Common Lane-Specific Artifacts
Beyond the classic edge effect, several other artifacts can manifest in specific lanes or across the gel due to sample-specific properties or running conditions:
- Smiling or Frowning Bands: Curved bands across the entire gel can result from uneven heat dissipation. "Smiling" bands (curving upward) occur when the center of the gel is hotter than the edges, causing faster migration in the middle. Conversely, "frowning" bands can form if the edges are warmer [64] [70].
- Band Smearing and Poor Resolution: This can be a lane-specific issue if caused by factors intrinsic to a single sample, such as high salt concentration or viscosity. High salt increases local conductivity and heating, leading to distorted bands that can affect neighboring lanes [64] [9]. Viscous samples, often from cell lysates containing genomic DNA, also lead to smeared migration patterns [9].
- Abnormal Migration Rates: A single lane migrating significantly faster or slower than others may indicate a problem with the sample's buffer composition, such as an incorrect ionic strength or pH [70].
Table 1: Troubleshooting Common Lane-Specific Artifacts in Denaturing Protein Gels
| Artifact Observed | Primary Cause | Impact on Data | Corrective Action |
|---|---|---|---|
| Distorted edge lanes | Non-uniform electric field; empty peripheral wells [71] [70] | Inaccurate molecular weight determination; invalid lane-to-lane comparison | Load control samples (e.g., ladder, buffer) in peripheral wells [70] |
| Lane-specific smearing | High salt concentration in the sample [64] [9] | Poor band resolution; inability to distinguish specific proteins | Desalt samples via dialysis or precipitation [9] |
| Lane-specific skewed bands | High viscosity due to genomic DNA in cell lysates [9] | Altered protein migration patterns; poor resolution | Shear genomic DNA by sonication or filtration [9] |
| Abnormal migration in one lane | Incorrect or depleted running buffer [64] [70] | Inaccurate size estimation; poor band resolution | Remake running buffer with correct ionic strength and pH [70] |
Quantitative Analysis of Artifact Severity
To systematically address these artifacts, it is essential to quantify their severity. Image analysis software can measure band distortion and lane-to-lane variation, providing objective metrics for troubleshooting efficacy. These tools can perform background correction, reduce noise, and quantify band profiles to precisely measure parameters like band curvature and migration distance [72].
Table 2: Quantitative Metrics for Assessing Gel Artifacts
| Metric | Description | Application | Measurement Tool |
|---|---|---|---|
| Band Curvature Index | Measures the deviation of a band from a perfectly straight line. | Quantifying the severity of "smiling" or "frowning" and the edge effect [64] [70]. | Densitometry profile analysis from gel imaging software [72]. |
| Lane Migration Variance | Calculates the standard deviation of migration distances for a standard protein across all lanes. | Assessing overall gel uniformity and the success of corrective protocols. | Molecular weight ladder analysis. |
| Signal-to-Noise Ratio | Quantifies the intensity of a specific band relative to the background smear. | Evaluating the effectiveness of protocols in reducing smearing and improving clarity [73]. | Background-corrected density profiling [72]. |
Experimental Protocols for Mitigation
Protocol 1: Preventing the Edge Effect
This protocol ensures a uniform electric field for consistent migration across all lanes.
Materials:
- Protein samples and molecular weight ladder
- SDS-PAGE gel apparatus and power supply
- Running buffer (e.g., Tris-Glycine-SDS)
Procedure:
- Gel Loading Strategy: When loading your gel, never leave the outermost wells empty. Instead, load an equal volume of 1X SDS-PAGE loading buffer or a control protein sample into these peripheral wells [70]. This ensures a uniform buffer interface and electric field strength across the entire gel.
- Apparatus Setup: Assemble the gel apparatus according to the manufacturer's instructions, ensuring the gel is properly seated and the electrodes are straight and clean [64].
- Buffer Volume: Fill the upper and lower buffer chambers with fresh running buffer to the recommended levels, ensuring the level is consistent across the gel tank [64].
- Electrophoresis Conditions: Run the gel at a constant voltage as recommended for the gel percentage. To minimize heat-related distortion, consider using a constant current power supply, which helps maintain a more uniform temperature [64]. If available, run the gel in a cold room or use a built-in cooling apparatus.
Protocol 2: Correcting for Sample-Specific Issues
This protocol addresses artifacts arising from problematic sample composition.
Materials:
- Cell lysis buffer (e.g., RIPA buffer)
- Protease inhibitor cocktail
- Benzonase nuclease or DNase I (optional)
- Dialysis kit or precipitation kit (e.g., acetone precipitation)
- Sonicator or needle and syringe
Procedure:
- Reducing Sample Viscosity:
- After cell lysis, centrifuge the lysate at >10,000 x g for 10 minutes to pellet insoluble debris, including genomic DNA [9].
- Transfer the soluble fraction to a new tube. If the sample remains viscous, shear the genomic DNA by briefly sonicating the sample (e.g., 3 pulses of 10 seconds on ice) or by passing it through a narrow-gauge needle (e.g., 27-gauge) 10-15 times [32].
- Alternatively, add Benzonase nuclease (a non-specific nuclease) to the lysate to digest all nucleic acids.
- Desalting Samples:
- For samples with high salt concentrations (e.g., from purification elution buffers), perform dialysis against a low-salt buffer or use a protein precipitation and resuspension method [9].
- For acetone precipitation, add 4 volumes of cold acetone to 1 volume of sample, incubate at -20°C for 1 hour, centrifuge to pellet the protein, and resuspend the air-dried pellet in 1X SDS-PAGE loading buffer.
- Ensuring Proper Denaturation:
- Mix the protein sample with an equal volume of 2X SDS-PAGE loading buffer containing a reducing agent (e.g., 50 mM DTT or 5% β-mercaptoethanol) [9].
- Heat the samples at 85°C for 2-5 minutes, not 100°C, to achieve complete denaturation without risking proteolysis [9].
- Centrifuge heated samples briefly to bring down condensation before loading the gel.
The Scientist's Toolkit: Essential Reagent Solutions
Table 3: Key Research Reagent Solutions for Artifact-Free Electrophoresis
| Reagent | Function | Protocol Note |
|---|---|---|
| SDS-PAGE Loading Buffer | Denatures proteins and provides dye for tracking migration. Contains SDS, glycerol, and a tracking dye. | Always include a reducing agent (e.g., DTT) for reduced samples. Add fresh before heating [9]. |
| Tris-Glycine-SDS Running Buffer | Maintains pH and provides ions for conductivity during electrophoresis. | Prepare fresh or use stored aliquots to avoid depletion of ions and pH shifts [64] [70]. |
| Protease Inhibitor Cocktail | Prevents protein degradation during sample preparation. | Add to lysis buffer immediately before use to preserve sample integrity [32]. |
| Benzonase Nuclease | Digests DNA and RNA to reduce sample viscosity. | Add to lysate according to manufacturer's instructions; requires Mg²⁺ for activity. |
| Dithiothreitol (DTT) | Reducing agent that breaks disulfide bonds for complete protein denaturation. | Use at a final concentration of 50 mM. Prepare fresh and add to sample just before heating [9]. |
Workflow Visualization
The following diagram illustrates the logical workflow for diagnosing and addressing the primary lane-specific artifacts discussed in this note.
Artifact Diagnosis and Resolution Workflow
Within the critical framework of sample preparation for denaturing gel electrophoresis, proactive management of the electrophoretic process itself is non-negotiable. The edge effect and related lane-specific artifacts are not mere inconveniences but significant sources of experimental variability that can invalidate comparative analyses. By integrating the systematic troubleshooting approaches, refined protocols, and quantitative assessments outlined in this document, researchers and drug development professionals can significantly enhance the reproducibility, reliability, and interpretability of their protein electrophoresis data, thereby strengthening the foundation of their scientific conclusions.
Within the critical workflow of denaturing protein gel electrophoresis, the clarity of bands on the resulting gel is a direct indicator of experimental success. Faint or absent bands represent a significant failure point, halting research and delaying drug development pipelines. This application note addresses this central problem by framing it within the broader context of sample preparation integrity. For researchers and scientists, the issues of sample loss and loading errors are not merely minor setbacks but are primary contributors to data loss, often stemming from subtle yet critical oversights in handling, preparation, and loading techniques [32] [58]. The following sections provide a detailed, actionable guide to diagnosing, troubleshooting, and preventing these common issues, ensuring the reliability of experimental data.
Core Concepts and Definitions
Understanding the fundamental causes of faint or absent bands is the first step toward mitigation. The problem can be systematically broken down into two primary categories:
- Sample Loss: This occurs when the target protein is degraded, improperly precipitated, or otherwise lost before it is loaded into the gel. The result is an insufficient quantity of intact protein to form a visible band [32] [64].
- Loading Errors: These are procedural mistakes that prevent the prepared sample from being successfully transferred from the tube into the gel matrix. Even a perfectly prepared sample will yield no result if it is not loaded correctly [63] [64].
The visual outcome on the gel can often point to the root cause. A complete absence of bands, including the loading control or molecular weight marker, typically indicates a gross loading error or an electrophoresis system failure [64]. In contrast, faint bands specifically in sample lanes, alongside a normal-appearing marker, strongly suggest issues of sample loss or degradation [63].
Root Cause Analysis
A systematic investigation into the origins of sample loss and loading errors reveals several critical failure points. The flowchart below maps the logical relationship between these causes and their observable effects, providing a diagnostic pathway.
Detailed Causes of Sample Loss
- Protease Activity: A primary cause of sample degradation. Proteases present in the original lysate can remain active in the sample buffer until the heating step denatures them. A delay of even a few minutes between mixing the sample with the buffer and heating can allow for significant protein degradation [58]. Using an inactive or insufficient protease inhibitor cocktail exacerbates this issue [32].
- Improper Sample Handling: Repeated freeze-thaw cycles of protein samples can lead to aggregation and precipitation, effectively reducing the concentration of soluble protein [32]. Furthermore, overly vigorous pipetting or vortexing of high molecular weight DNA or protein samples can cause mechanical shearing, fragmenting the molecules and resulting in a smear or loss of a distinct band [32].
- Incorrect Buffer-to-Protein Ratio: The sample buffer must contain a sufficient excess of SDS to coat all proteins in the sample consistently. Hames [58] recommends a mass ratio of at least 3:1 (SDS to protein) to ensure complete denaturation and charge masking. Inadequate SDS leads to incomplete denaturation and poor migration into the gel [58].
Detailed Causes of Loading Errors
- Insufficient Sample Concentration: A fundamental error is loading a total protein mass that is below the detection limit of the subsequent staining method. For Coomassie Blue staining, 0.5–4.0 µg of a purified protein is typically required, while silver staining, being more sensitive, requires less [58]. Inaccurate quantification, often due to using an incompatible assay (e.g., BCA vs. Bradford), can lead to loading an unknowingly low amount of protein [32].
- Well Damage or Leakage: Poorly formed wells can cause the sample to leak out into the surrounding buffer before it enters the gel. This can occur if the gel comb is pushed all the way to the bottom of the cassette or if the comb is removed before the gel is fully polymerized [63]. Overloading the physical volume of the well can also cause sample spillover.
- Power Supply and Connection Issues: Simple mistakes, such as incorrect electrode connection (reversed polarity) or a power supply that was not properly engaged, will prevent current from flowing and samples from migrating into the gel [63] [64]. A short circuit in the system can also cause this failure.
Quantitative Data and Parameters
Successful sample preparation hinges on adhering to specific quantitative benchmarks. The following tables summarize the critical parameters for avoiding sample loss and loading errors.
Table 1: Sample Preparation Parameters to Prevent Loss
| Parameter | Optimal Value or Condition | Rationale & Consequences of Deviation |
|---|---|---|
| Protease Inhibitor Addition | Added immediately to lysis buffer [32]. | Rationale: Inactivates proteases upon cell lysis.Deviation: Delay leads to sample degradation and smeared/faint bands. |
| Heating Step Post-Buffer | Immediately after mixing with sample buffer [58]. | Rationale: Instantly denatures proteases and proteins.Deviation: Room temperature incubation allows proteolysis. |
| Heating Temperature/Time | 75°C for 5 min [58] OR 95-100°C for 5 min [58]. | Rationale: 75°C avoids Asp-Pro bond cleavage. 100°C ensures full denaturation for most proteins. |
| SDS-to-Protein Ratio | Minimum 3:1 mass ratio [58]. | Rationale: Ensures complete protein coating for uniform charge.Deviation: Poor migration, band distortion. |
| Sample Concentration | 0.5-4 µg (purified protein, Coomassie) 40-60 µg (crude sample, Coomassie) [58]. | Rationale: Ensures band is within linear detection range.Deviation: Under-loading causes faint bands; over-loading causes smearing. |
Table 2: Electrophoresis Parameters to Prevent Loading Errors
| Parameter | Optimal Value or Condition | Rationale & Consequences of Deviation |
|---|---|---|
| Well Volume Utilization | Fill at least 30% of well volume [63]. | Rationale: Ensures sample sinks properly into well.Deviation: Low volume can lead to diffusion and poor entry. |
| Well Integrity | Do not push comb to very bottom of cassette [63]. | Rationale: Prevents sample leakage under the gel.Deviation: Sample lost to running buffer. |
| Electrode Polarity | Gel wells on cathode (negative) side [63]. | Rationale: Proteins are negatively charged in SDS-PAGE and migrate toward anode.Deviation: Sample migrates wrong way or not at all. |
| Post-Heating Centrifugation | Brief spin (e.g., 2 min at 17,000 x g) [58]. | Rationale: Pellets insoluble debris that can cause streaking.Deviation: Insoluble material loaded, clogging well. |
Detailed Experimental Protocols
Protocol 1: Optimized Denaturing Sample Preparation
This protocol is designed to minimize sample loss through degradation or improper denaturation.
Title: Preparation of Protein Samples for Denaturing SDS-PAGE.
Principle: Proteins are denatured, reduced, and coated with the anionic detergent SDS to impart a uniform negative charge, allowing separation based primarily on molecular weight.
Reagents:
- Lysis Buffer (e.g., RIPA Buffer)
- 4X Laemmli Sample Buffer: 250 mM Tris-HCl (pH 6.8), 8% SDS, 40% Glycerol, 20% β-Mercaptoethanol, 0.02% Bromophenol Blue.
- Fresh Protease Inhibitor Cocktail (PIC)
- BCA or Bradford Protein Assay Reagents
Procedure:
- Lysis: Lyse cells or tissue in an appropriate volume of ice-cold lysis buffer supplemented with PIC. Incubate on ice for 15-30 minutes with gentle agitation.
- Clarification: Centrifuge the lysate at >12,000 x g for 15 minutes at 4°C to remove insoluble material. Transfer the supernatant to a new tube.
- Quantification: Determine the protein concentration of the supernatant using a compatible protein assay (e.g., BCA assay).
- Sample Buffer Addition: Dilute the protein extract with 4X Laemmli Sample Buffer to achieve a 1X final concentration. Ensure the SDS-to-protein ratio is at least 3:1 [58]. Mix thoroughly by pipetting.
- Immediate Denaturation: Immediately after mixing, heat the samples at 75°C for 5 minutes (to avoid Asp-Pro cleavage) or 95-100°C for 5 minutes [58].
- Brief Centrifugation: Pulse-centrifuge (e.g., 30 seconds at 17,000 x g) to collect condensation and any insoluble aggregates.
- Loading: Load the recommended volume (based on quantification) onto the gel. Store unused, prepared samples at -20°C or -80°C. Avoid multiple freeze-thaw cycles.
Protocol 2: Systematic Checks for Loading and Run Failure
This procedural checklist diagnoses and corrects loading and electrophoresis runtime failures.
Title: Diagnostic Checklist for Absent Bands.
Principle: Methodically verify each step of the loading and electrophoresis process to isolate the point of failure.
Procedure:
- Verify Marker Bands:
- Observation: No bands in any lane, including the molecular weight marker.
- Diagnosis: System-wide loading or electrophoresis failure.
- Action: Proceed to Step 2.
- Observation: Marker bands are present, but sample bands are faint/absent.
- Diagnosis: Sample-specific issue (degradation, low concentration).
- Action: Troubleshoot sample preparation (see Protocol 1).
Check Power Supply & Connections:
- Confirm the power supply is turned ON and outputting the correct voltage/current.
- Verify the electrodes are connected with the correct polarity (black/- to upper buffer chamber/cathode for standard SDS-PAGE).
- Inspect the tank and wires for signs of a short circuit.
Inspect Gel and Wells:
- Examine wells for damage caused during comb removal or pipetting [63].
- Confirm the sample was loaded into the well and did not spill over into an adjacent well.
- Ensure the gel is fully submerged in the running buffer and that the buffer level is consistent.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Preventing Sample Loss
| Reagent | Function & Rationale |
|---|---|
| Protease Inhibitor Cocktail (PIC) | A mixture of inhibitors that target various classes of proteases (serine, cysteine, metallo-, etc.). Critical for preventing co-purified proteases from degrading the target protein during and after lysis [32]. |
| SDS (Sodium Dodecyl Sulfate) | An ionic detergent that denatures proteins and binds in a constant mass ratio, imparting a uniform negative charge. An excess (≥3:1 SDS:protein ratio) is required for complete denaturation and sharp bands [58]. |
| DTT (Dithiothreitol) or β-Mercaptoethanol | Reducing agents that break intramolecular and intermolecular disulfide bonds. This ensures proteins are fully unfolded and migrate as individual polypeptides, preventing aberrant migration and smearing [58]. |
| BCA Protein Assay Kit | A colorimetric method for quantifying total protein concentration. More compatible with detergents common in lysis buffers than the Bradford assay, leading to more accurate quantification and, thus, more precise loading [32]. |
| High-Purity, Nuclease-Free Water | Used to prepare all buffers and solutions. Prevents introduction of contaminants or nucleases that could interfere with the assay or degrade samples [63] [32]. |
Optimization Checklist for Sharp, Publication-Quality Bands
In denaturing protein gel electrophoresis research, the quality of the final result is determined at the sample preparation stage. Publication-quality results with sharp, well-resolved bands are not a product of electrophoresis alone but of a meticulously optimized pre-analytical workflow. This application note, framed within a broader thesis on sample preparation, provides researchers, scientists, and drug development professionals with a detailed, actionable checklist and supporting protocols. The goal is to standardize procedures, minimize variability, and ensure that the data generated is reliable, reproducible, and worthy of scientific trust. The foundation of this process is the recognition that sample integrity is paramount; even the most advanced electrophoresis equipment cannot compensate for a degraded or improperly prepared sample [32].
The Scientist's Toolkit: Essential Reagents and Materials
Successful SDS-PAGE relies on a set of core reagents, each fulfilling a specific role in denaturing, stabilizing, and tracking the protein sample during separation.
Table 1: Key Research Reagent Solutions for Denaturing Protein Gel Electrophoresis
| Reagent/Material | Function/Purpose | Key Considerations |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) | Denatures proteins and confers a uniform negative charge, enabling separation primarily by molecular weight [74] [75]. | Use a high-purity grade; ensure final concentration in sample buffer is sufficient to fully coat proteins. |
| Reducing Agents (DTT, β-mercaptoethanol, TCEP) | Breaks disulfide bonds to fully linearize protein subunits [9] [74]. | Prepare fresh or store properly; TCEP is more stable and resistant to oxidation than DTT or β-mercaptoethanol [9]. |
| Protease Inhibitor Cocktails | Prevents proteolytic degradation of the target protein during cell lysis and sample preparation [32]. | Must be added fresh to the lysis buffer immediately before use to maintain activity. |
| Lysis Buffers | Solubilizes proteins from cells or tissues. | Composition (e.g., detergent, salt) should be compatible with the sample type and downstream analysis. |
| Laemmli Sample Buffer | A standard buffer containing SDS, glycerol, a tracking dye, and a buffer to prepare the sample for loading [74]. | The glycerol adds density for loading; the dye (e.g., bromophenol blue) allows visual tracking of the run. |
| Pre-stained Protein Ladder | Provides molecular weight standards for estimating protein size and monitoring electrophoresis progress [74]. |
Optimization Checklist & Quantitative Parameters
The following checklist and associated tables summarize the critical parameters that must be controlled to achieve sharp, publication-quality bands.
Comprehensive Optimization Checklist
Sample Preparation
- Use a fresh, appropriate reducing agent and avoid long-term storage of reduced samples [9].
- Denature samples at 85°C for 2–5 minutes; avoid 100°C to prevent proteolysis [9].
- For cell lysates, shear genomic DNA to reduce viscosity and clear lysates by centrifugation [9] [32].
- Mitigate high salt concentrations or guanidine-HCl by dialysis or precipitation to prevent increased conductivity and artifacts [9].
- Accurately quantify protein concentration using a BCA or Bradford assay to ensure equal loading [32].
- Include protease inhibitors during lysis and keep samples on ice to prevent degradation [32].
Gel Loading & Electrophoresis
- Load an equal and appropriate sample volume (e.g., ~10 µL for a standard mini-gel well) [76].
- Load a pre-stained protein ladder in one lane for molecular weight estimation [74].
- Run gels using a step-voltage protocol: start at ~80V until samples enter the resolving gel, then increase to ~120V [76].
- Use fresh electrophoresis buffer for optimal results; buffer can typically be reused 1-2 times [76].
- Stop the run when the dye front (e.g., bromophenol blue) reaches the bottom of the gel [76].
Table 2: Key Quantitative Parameters for Optimized SDS-PAGE
| Parameter | Optimal Value or Range | Purpose and Rationale |
|---|---|---|
| Reducing Agent | 50 mM DTT or 50 mM TCEP [9] | Fully breaks disulfide bonds for complete linearization. |
| Denaturation | 85°C for 2-5 minutes [9] | Unfolds proteins without inducing proteolysis. |
| Sample Volume | ~10 µL per well (mini-gel) [76] | Prevents overloading and well overflow. |
| Initial Voltage | 80 V [76] | Allows slow, even entry into the resolving gel for sharp bands. |
| Main Voltage | 120 V [76] | Accelerates separation after stacking is complete. |
| Run Time | 80-90 minutes (for 10-12% gels) [76] | Ensures adequate separation without losing small proteins. |
Detailed Experimental Protocols
Protocol: Preparation of Denatured and Reduced Protein Samples
This protocol is designed for standard cell lysates or purified protein samples destined for SDS-PAGE analysis.
Materials:
- Laemmli Sample Buffer (e.g., 4X concentration)
- Fresh Reducing Agent (e.g., 0.5M DTT stock or 0.5M TCEP stock)
- Protein Sample (quantified)
- Thermonixer or heating block
Method:
- Dilute and Mix: Combine the protein sample with an appropriate volume of Laemmli sample buffer to achieve a 1X final concentration. For example, for 30 µL of protein sample, add 10 µL of 4X sample buffer.
- Add Reducing Agent: Add a fresh reducing agent to the sample-buffer mixture. For a final concentration of 50 mM, add 1 µL of a 0.5M DTT or TCEP stock for every 9 µL of the sample-buffer mixture [9].
- Denature: Heat the mixture at 85°C for 2-5 minutes [9]. Do not exceed this temperature or duration to avoid protein degradation.
- Brief Centrifugation: Briefly spin the tube in a microcentrifuge to collect all condensation and liquid at the bottom of the tube.
- Load or Store: Load the sample immediately onto the gel. If storage is necessary, flash-freeze in liquid nitrogen and store at -80°C. Avoid multiple freeze-thaw cycles, as reoxidation can occur and produce inconsistent results [9].
Protocol: Optimized SDS-PAGE Electrophoresis Run
This protocol assumes a standard polyacrylamide mini-gel (e.g., 1.0 mm thick) has been cast and assembled in a vertical electrophoresis cell.
Materials:
- Prepared polyacrylamide gel
- Pre-stained protein molecular weight marker
- Prepared protein samples
- SDS-PAGE Running Buffer (e.g., Tris-Glycine-SDS)
- Power supply
Method:
- Assemble and Fill: Place the gel cassette into the electrophoresis tank and fill the inner and outer chambers with running buffer, ensuring the wells are submerged.
- Load Samples: Using a gel-loading pipette tip, carefully load the recommended volume (e.g., 10-20 µL) of the prepared protein samples and molecular weight marker into the designated wells [76].
- Run with Step-Voltage:
- Initial Phase: Attach the lid to the tank, connecting the electrodes correctly (black to cathode, red to anode). Set the power supply to a constant voltage of 80V and start the run [76].
- Transition: Continue at 80V until the bromophenol blue dye front has completely entered and formed a thin line at the top of the resolving gel. This typically takes 15-20 minutes.
- Main Phase: Increase the voltage to 120V for the remainder of the run [76].
- Monitor and Stop: Run the gel until the dye front just begins to exit the bottom of the gel. For a 10-12% gel, this typically takes a total of 80-90 minutes [76].
- Proceed to Staining: Once the run is complete, turn off the power supply, disassemble the apparatus, and carefully open the cassette to retrieve the gel for staining (e.g., with Coomassie Blue) or downstream processing like Western blotting [75].
Troubleshooting Common Issues
Even with careful optimization, issues can arise. The table below outlines common problems, their likely causes, and recommended solutions.
Table 3: Troubleshooting Guide for Common SDS-PAGE Issues
| Problem | Potential Causes | Solutions |
|---|---|---|
| Smearing Bands | - Protein degradation [32]- Overloaded sample [32]- Incomplete denaturation | - Use fresh protease inhibitors [32].- Load less protein.- Ensure fresh reducing agent and correct heating. |
| Vertical Streaks | - Presence of genomic DNA in lysates [9]- Insoluble material loaded | - Shear DNA by sonication or pass lysate through a fine-gauge needle [9] [32].- Centrifuge lysate and load only soluble fraction. |
| Uneven or Wavy Bands | - High salt concentration in sample [9]- Crystallized or precipitated SDS in the gel | - Dialyze sample or perform protein precipitation to desalt [9].- Ensure SDS is fully dissolved in all buffers. |
| Poor Band Resolution | - Incorrect gel concentration [32]- Run time too short or too long- Voltage too high | - Use a higher % gel for smaller proteins, lower % for larger proteins [32].- Adjust run time so dye front nears but does not run off.- Follow step-voltage protocol for optimal stacking and resolution [76]. |
Validating Your Results: Ensuring Accuracy and Preparing for Downstream Applications
Using Protein Ladders and Molecular Weight Markers for Validation
In the context of denaturing protein gel electrophoresis research, the validation of experimental results is paramount. Molecular weight markers, often referred to as protein ladders or standards, are indispensable tools for this purpose. These standards consist of a mixture of highly purified proteins with known molecular weights, which are separated during electrophoresis to provide a reference scale for estimating the size of unknown proteins in adjacent lanes [77] [78]. Their function extends beyond mere size determination; they are critical controls for verifying the proper progression of electrophoretic separation, assessing protein transfer efficiency in western blotting, and confirming the overall integrity of the experimental workflow [79].
The choice between different types of markers—such as prestained versus unstained, or broad range versus high range—is dictated by the specific application and the required balance between convenience and precision. Prestained markers allow for real-time monitoring of protein migration during SDS-PAGE and transfer efficiency during western blotting, as their colored bands can be visualized directly [79] [78]. In contrast, unstained markers provide superior accuracy for molecular weight determination because the attached dyes in prestained markers can alter protein mobility, leading to slight deviations in apparent molecular weight [79] [78]. Understanding these characteristics is fundamental to selecting the appropriate validation tool for denaturing gel electrophoresis.
Selection Guide for Protein Standards
Choosing the correct protein standard is a critical step in experimental design, as the appropriate marker enhances reliability while an inappropriate one can lead to misinterpretation. The selection can be broadly categorized into molecular weight markers and protein ladders. Protein ladders are composed of a set of highly purified recombinant proteins whose sizes correspond to precise, whole-number values (e.g., 10, 15, 25 kDa, etc.), providing a precise scale for size estimation. In contrast, traditional molecular weight markers are mixtures of native proteins with well-characterized but not necessarily uniformly spaced molecular weights, often making them a more economical choice for approximate sizing [78].
The experimental application heavily influences the choice. For routine SDS-PAGE where monitoring migration is sufficient, prestained markers are ideal. For western blotting, specialized markers with IgG-binding sites on some or all bands (e.g., MagicMark XP Western Protein Standard) enable direct visualization on the blot membrane upon antibody detection, serving as a positive control for the detection system [79]. For precise molecular weight determination, unstained standards visualized by protein stains like Coomassie Blue offer the highest accuracy, as they are free from the mobility-shift artifacts caused by dye conjugation [79] [78]. Furthermore, specialized ladders are available for applications such as analyzing phosphoproteins, glycoproteins, or His-tagged proteins [79].
Table 1: Comparison of Prestained and Unstained Protein Markers
| Feature | Prestained Markers | Unstained Markers |
|---|---|---|
| Primary Use | Monitoring electrophoresis and transfer | Accurate molecular weight determination |
| Visualization | Direct, colorimetric during/after run | Requires post-staining (e.g., Coomassie) |
| Size Accuracy | Lower (dye alters migration) | Higher |
| Western Blotting | Can monitor transfer; some are blottable | Can be detected with specific tags (e.g., Strep-tag) |
| Examples | PageRuler Plus Prestained, Spectra Multicolor [79] | PageRuler Unstained, HiMark Unstained [79] |
Table 2: Selection of Protein Ladders for Specific Applications
| Application | Recommended Marker Type | Key Characteristics | Product Example |
|---|---|---|---|
| Routine SDS-PAGE | Prestained Broad Range | Multicolored bands for easy tracking | PageRuler Plus Prestained (10-250 kDa) [79] |
| Precise MW Determination | Unstained Broad Range | High accuracy, requires staining | PageRuler Unstained (10-200 kDa) [79] |
| High MW Proteins | Prestained/Unstained High Range | Optimized for large proteins | HiMark Prestained (31-460 kDa) [79] |
| Western Blot Positive Control | IgG-Binding Western Standard | Bands detected during immunodetection | MagicMark XP (20-220 kDa) [79] |
| His-Tagged Protein Analysis | Unstained His-Tagged Standard | Bands contain a 6X His-tag for detection | BenchMark His-tagged (10-160 kDa) [79] |
The following decision pathway outlines the process for selecting the appropriate molecular weight marker based on experimental requirements.
Detailed Experimental Protocols
Protocol 1: SDS-PAGE with Prestained Protein Ladders
This protocol outlines the steps for performing denaturing SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using a prestained protein ladder for validation of protein separation and size estimation [79].
Research Reagent Solutions
- Prestained Protein Ladder: A ready-to-use mixture of recombinant proteins pre-coupled to dyes (e.g., PageRuler Plus Prestained). Provides visual monitoring of electrophoresis and transfer. [79]
- SDS-PAGE Gel: A polyacrylamide gel cast with SDS (sodium dodecyl sulfate). The percentage (e.g., 4-20%) determines the resolution range.
- Running Buffer: Typically Tris-Glycine-SDS buffer, pH 8.3. Provides the ions for conductivity and maintains pH for proper migration.
- Laemmli Sample Buffer: Contains SDS, glycerol, a reducing agent (e.g., DTT), and a tracking dye. Denatures proteins and confers a uniform negative charge.
- Protein Samples: The target proteins of interest, diluted in or mixed with sample buffer.
Procedure
- Sample Preparation: Mix protein samples with an equal volume of 2X Laemmli sample buffer. Heat the samples at 70-95°C for 5-10 minutes to fully denature the proteins. Briefly centrifuge to collect condensation.
- Gel Preparation: Assemble the gel electrophoresis unit according to the manufacturer's instructions. Fill the inner and outer chambers with running buffer to submerge the gel wells.
- Loading: Using a pipette, load 5 µL of the prestained protein ladder into the first well of a 1.0 mm mini-gel. Load equal volumes of prepared protein samples into adjacent wells [79].
- Electrophoresis: Connect the power supply, applying a constant voltage. A typical setting is 120-150 V for a mini-gel. Run the gel until the dye front (typically bromophenol blue) has migrated to the bottom of the gel.
- Visualization: Upon completion, the colored bands of the prestained ladder will be visible. The gel can be further processed for staining (e.g., Coomassie, silver stain) or for western blot transfer.
Protocol 2: Western Blot Validation with IgG-Binding Protein Standards
This protocol details the use of specialized protein standards for validating transfer efficiency and providing a molecular weight reference directly on the western blot membrane [79].
Research Reagent Solutions
- IgG-Binding Western Standard: A prestained ladder (e.g., MagicMark XP) where all bands are recombinant proteins fused to an IgG-binding domain. Allows immunodetection on the blot. [79]
- Transfer Buffer: A buffer such as Tris-Glycine with methanol, used for wet or semi-dry protein transfer from the gel to a membrane.
- Membrane: Nitrocellulose or PVDF membrane, which binds proteins.
- Blocking Buffer: A solution of 5% non-fat dry milk or BSA in TBST to prevent non-specific antibody binding.
- Primary and Secondary Antibodies: Target-specific and enzyme-conjugated antibodies for detection.
Procedure
- Electrophoresis and Transfer: Perform SDS-PAGE as described in Protocol 3.1, but load 5-10 µL of the IgG-binding standard alongside your samples [79]. Following electrophoresis, transfer the proteins from the gel onto a membrane using standard wet or semi-dry transfer protocols.
- Post-Transfer Check: After transfer, visually inspect the membrane. The colored bands of the standard will confirm successful transfer from the gel to the membrane.
- Blocking: Incubate the membrane in blocking buffer for 1 hour at room temperature with gentle agitation.
- Immunodetection: Incubate the membrane with the primary antibody (diluted in blocking buffer) as per the specific protocol, followed by the appropriate enzyme-conjugated secondary antibody.
- Detection and Validation: During the final chemiluminescent or colorimetric detection step, the bands of the IgG-binding standard will become visible alongside the target protein bands. This provides an internal molecular weight reference on the final blot image and serves as a positive control for the detection system.
Validation in Broader Research Context
Within a comprehensive thesis on sample preparation for denaturing gel electrophoresis, the consistent and correct use of protein ladders serves as a cornerstone of methodological rigor. The validation they provide is not an isolated step but is integrated throughout the research process. For instance, in drug development, the confirmation of a recombinant therapeutic protein's size and purity is a critical quality control checkpoint, and this relies heavily on accurate calibration with appropriate molecular weight standards [79].
Furthermore, the choice of marker can validate specific sample preparation steps. The use of an unstained, recombinant ladder can confirm that the sample buffer and reducing conditions were effective in denaturing proteins without introducing artifacts. Specialized ladders, such as phosphoprotein or glycoprotein standards, can be run alongside experimental samples to validate the effectiveness of enrichment protocols or specific detection methods [79]. By systematically incorporating these standards into every electrophoresis run, researchers can distinguish between true experimental results and potential artifacts arising from sample degradation, incomplete transfer, or inefficient detection, thereby ensuring the integrity and reproducibility of their scientific findings.
Within the broader context of sample preparation for denaturing protein gel electrophoresis research, the assessment of sample purity and concentration is a critical prerequisite for experimental success. Accurate quantification and integrity analysis are fundamental to obtaining reliable, reproducible results in downstream applications such as immunoblotting or mass spectrometry. This application note details two cornerstone methodologies—spectrophotometry and gel electrophoresis—for evaluating protein samples, providing structured protocols and comparative analyses to guide researchers in selecting and implementing the appropriate technique for their experimental needs.
Analytical Methods for Purity and Quantity Assessment
Spectrophotometry: Principles and Applications
Spectrophotometry provides a rapid, quantitative assessment of protein concentration and purity by measuring the absorption of ultraviolet (UV) light at specific wavelengths. The principle is based on the characteristic absorption maxima of proteins and common contaminants [80].
Concentration Calculation: Protein concentration is determined by measuring absorbance at 280 nm (A₂₈₀), primarily due to tyrosine, tryptophan, and phenylalanine residues. The specific absorption coefficient for a 1 mg/mL solution of a standard protein is approximately 1.0 AU, though this varies significantly between proteins. The general formula for concentration is: Concentration (mg/mL) = (A₂₈₀ reading – A₃₂₀ reading) × dilution factor × correction factor [80].
Purity Ratios: The integrity of a protein sample is evaluated using absorbance ratios [80] [81]:
- A₂₆₀/A₂₈₀: This ratio detects nucleic acid contamination. A pure protein sample typically has a ratio of approximately 0.6. A ratio higher than 0.8 suggests significant nucleic acid presence.
- A₂₆₀/A₂₃₀: This ratio indicates the presence of chaotropic salts, buffers, or other chemical contaminants. A pure protein sample typically exhibits a ratio greater than 1.5 [80].
For the most informative profile, a full spectral scan from 230 nm to 320 nm is recommended, with the A₃₂₀ reading used to correct for turbidity or light scattering [80].
Gel Electrophoresis: Principles and Applications
Denaturing gel electrophoresis, typically using SDS-PAGE, provides a qualitative and semi-quantitative visual assessment of protein sample integrity, complexity, and approximate molecular weight [82]. Under denaturing conditions, proteins are separated based on their molecular weight, allowing researchers to confirm the presence of a target band, identify degradation (evidenced by smearing), and detect the presence of contaminating proteins or unexpected post-translational modifications [81].
Table 1: Comparison of Protein Assessment Methods
| Parameter | Spectrophotometry | Gel Electrophoresis |
|---|---|---|
| Primary Output | Quantitative concentration & purity ratios | Qualitative/Semi-quantitative integrity & complexity |
| Sample Throughput | High (seconds per sample) | Low (hours per run) |
| Information Gained | Nucleic acid & chemical contamination | Molecular weight, degradation, contaminating proteins |
| Key Advantage | Speed, quantitative data | Visual integrity check, separation of components |
| Main Limitation | Cannot distinguish intact from degraded protein; contaminant interference [83] | Semi-quantitative, time-consuming, requires optimization |
Experimental Protocols
Protocol A: Spectrophotometric Analysis of Protein Samples
This protocol is adapted from principles used in nucleic acid quantification and tailored for protein analysis [80].
I. Materials and Reagents
- UV-transparent cuvette (e.g., quartz)
- Protein sample in a compatible, non-absorbing buffer (e.g., PBS)
- Spectrophotometer with UV lamp
- Dilution buffer (matches sample buffer)
II. Procedure
- Instrument Calibration: Blank the spectrophotometer using the buffer in which the protein is dissolved.
- Dilution: Dilute the protein sample to ensure absorbance readings fall within the instrument's linear range (typically A₂₈₀ between 0.1 and 1.0).
- Absorbance Measurement:
- Measure and record absorbance at 320 nm (A₃₂₀) for turbidity correction.
- Measure absorbance at 230 nm (A₂₃₀), 260 nm (A₂₆₀), and 280 nm (A₂₈₀).
- Data Calculation:
- Corrected A₂₈₀ = (A₂₈₀ reading – A₃₂₀ reading)
- Protein Concentration = Corrected A₂₈₀ × dilution factor × correction factor*
- Purity Ratios: Calculate A₂₆₀/A₂₈₀ and A₂₆₀/A₂₃₀ using corrected values.
*The correction factor is protein-specific. Use 1.0 for a standard protein like BSA or the theoretical extinction coefficient for your protein of interest.
Protocol B: Denaturing Gel Electrophoresis for Protein Integrity Check
This protocol outlines the steps for SDS-PAGE analysis to visually assess protein sample quality [9].
I. Materials and Reagents
- Pre-cast or hand-cast SDS-polyacrylamide gel
- Protein molecular weight marker (ladder)
- SDS-PAGE running buffer (e.g., 1X Tris-Glycine-SDS)
- Protein sample
- Loading dye (Laemmli buffer with SDS and tracking dyes)
- Heating block or water bath
- Electrophoresis cell and power supply
II. Procedure
- Sample Preparation:
- Mix protein sample with an appropriate volume of 2X or 4X Laemmli buffer.
- For denatured samples, heat at 85°C for 2–5 minutes [9]. Do not heat samples for native (non-denaturing) electrophoresis.
- Briefly centrifuge to collect condensation.
Gel Loading and Running:
- Load molecular weight marker and prepared protein samples into wells.
- Run the gel at constant voltage (e.g., 100-150V) until the dye front nears the bottom.
Staining and Visualization:
- Following electrophoresis, stain the gel with a protein-specific stain (e.g., Coomassie Blue, silver stain, or SYPRO Ruby).
- Destain (if required) and image the gel using a standard gel documentation system.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Protein Purity Assessment
| Reagent / Material | Function / Application |
|---|---|
| UV-Transparent Cuvettes | Holds sample for accurate UV absorbance measurement in a spectrophotometer. |
| Compatible Elution Buffer | A low-salt, non-absorbing buffer for diluting and storing purified protein samples. |
| SDS-PAGE Gel | Polyacrylamide matrix for separating proteins by molecular weight under denaturing conditions. |
| Protein Molecular Weight Marker | A mixture of proteins of known sizes for estimating the molecular weight of sample proteins. |
| Laemmli Loading Buffer | Contains SDS to denature proteins and a dye to track migration during electrophoresis. |
| Reducing Agent (DTT, β-Mercaptoethanol) | Added to loading buffer to break disulfide bonds; use a 50 mM final concentration of DTT or 2.5% final concentration of β-mercaptoethanol [9]. |
| Protein Stain (Coomassie, Silver, etc.) | For visualizing protein bands on the gel after electrophoresis. |
Methodological Workflow and Data Integration
The following workflow diagram illustrates the decision-making process for assessing protein sample purity, integrating both spectrophotometry and gel analysis.
Protein Purity and Integrity Assessment Workflow
Robust assessment of protein sample purity and integrity via spectrophotometry and gel analysis is a non-negotiable step in the pipeline of denaturing gel electrophoresis research. While spectrophotometry offers a rapid, quantitative check for chemical contaminants and concentration, gel electrophoresis provides an indispensable visual confirmation of structural integrity. Used in tandem, these methods form a complementary QC framework that ensures the reliability of protein samples, thereby safeguarding the investment of time and resources in downstream analytical processes and ultimately contributing to the generation of high-quality, reproducible scientific data.
Comparing Sample Preparation Methods for Different Starting Materials (Tissues, Cultured Cells, Biofluids)
Sample preparation is a critical preanalytical step that directly influences the success and reproducibility of downstream denaturing protein gel electrophoresis. The diversity of biological starting materials—each with a unique matrix composition and biochemical properties—necessitates tailored preparation strategies to ensure optimal protein yield, purity, and compatibility with electrophoretic systems. This application note provides a structured comparison of optimized protocols for tissues, cultured cells, and biofluids, framed within the context of preparing denatured protein samples for polyacrylamide gel electrophoresis (PAGE). The methods detailed herein are designed to minimize variability, preserve protein integrity, and facilitate accurate protein quantification, thereby supporting robust scientific research and drug development.
Comparative Analysis of Sample Preparation Methods
The choice of sample preparation method is primarily dictated by the starting material. The table below summarizes the key characteristics, recommended lysis buffers, and primary challenges associated with processing tissues, cultured cells, and biofluids.
Table 1: Comparison of Sample Preparation Methods for Different Starting Materials
| Starting Material | Key Characteristics | Recommended Lysis Buffer | Key Challenges |
|---|---|---|---|
| Tissues | Heterogeneous cell types, dense extracellular matrix, higher enzymatic activity [36] | T-PER Reagent (mild, for protein-protein interactions) or RIPA Buffer (strong, for membrane-bound/nuclear proteins) [36] | Requires mechanical homogenization; high lipid and protease content [36] |
| Cultured Cells | Homogeneous population, simpler matrix, easier to lyse [36] | M-PER (mild, whole cell) or RIPA Buffer (stronger, for difficult-to-extract proteins) [36] | Rapid enzymatic degradation post-lysis; adherence to culture vessel [36] |
| Biofluids | Liquid matrix, variable viscosity and composition (e.g., proteins, salts, metabolites) [84] | Dilution (urine) or Protein Precipitation (plasma, serum) [84] | High abundance of interfering compounds (e.g., albumin, phospholipids); often requires analyte concentration [84] |
Detailed Experimental Protocols
Preparation of Lysate from Cultured Cells
This protocol is optimized for recovering proteins from adherent or suspension mammalian cell cultures for denaturing SDS-PAGE [36].
Materials
- Cell Lysis Buffer (e.g., RIPA or M-PER) [36]
- Protease and Phosphatase Inhibitor Cocktail (100X) [36]
- Ice-cold Phosphate-Buffered Saline (PBS) [36]
- LDS Sample Buffer (4X) [36]
- Sample Reducing Agent (10X) [36]
- BCA Protein Assay Kit [36]
Procedure
- Prepare Lysis Buffer: Add protease and phosphatase inhibitors to the chilled lysis buffer immediately before use (e.g., 10 µL of inhibitor cocktail per 1 mL of buffer) [36].
- Wash Cells: For adherent cells, place the culture dish on ice, aspirate the medium, and wash cells with ice-cold PBS. For suspension cells, pellet by centrifugation at 2,500 x g for 10 minutes and wash with PBS [36].
- Lyse Cells:
- Clarify Lysate: Centrifuge the lysate at ~14,000 x g for 15 minutes at 4°C. Transfer the supernatant (clarified lysate) to a new microcentrifuge tube [36].
- Determine Protein Concentration: Perform a BCA protein assay. Use BSA standards to generate a standard curve and determine the concentration of your samples [36].
- Prepare Sample for Electrophoresis: Mix the protein lysate with LDS sample buffer and reducing agent. A typical formulation for a reduced sample is [36]:
- Protein Sample: x µL
- LDS Sample Buffer (4X): 2.5 µL
- Reducing Agent (10X): 1 µL
- Deionized water: to a final volume of 10 µL Heat the samples at 70°C for 2-10 minutes to denature proteins [36].
Preparation of Lysate from Tissues
Tissue samples require an initial mechanical disruption step to achieve effective lysis [36].
Materials
- Tissue Lysis Buffer (e.g., T-PER or RIPA) [36]
- Protease and Phosphatase Inhibitor Cocktail [36]
- Ice-cold PBS [36]
- Homogenizer (e.g., Dounce, mechanical rotor-stator)
Procedure
- Prepare Lysis Buffer: Add protease and phosphatase inhibitors to chilled lysis buffer as described in section 3.1.2 [36].
- Dissect and Weigh: Dissect the tissue of interest on ice and weigh it. A ratio of 50 mg tissue to 1,000 µL of lysis buffer is a good starting point [36].
- Homogenize: Add the appropriate volume of ice-cold lysis buffer to the tissue and homogenize thoroughly on ice.
- Clarify Lysate: Centrifuge the homogenate at 10,000 x g for 5 minutes at 4°C to pellet insoluble debris. Transfer the supernatant to a new tube [36].
- Determine Concentration and Prepare Sample: Quantify protein concentration using the BCA assay and prepare the sample for electrophoresis as described in steps 5 and 6 of section 3.1.2 [36].
Preparation of Samples from Biofluids
Biofluids like plasma, serum, and urine present unique challenges and often require methods beyond simple lysis. The choice of technique depends on the desired analyte and the need to remove matrix interferents [84].
Table 2: Common Sample Preparation Techniques for Biofluids [84]
| Technique | Principle | Best For | Advantages | Disadvantages |
|---|---|---|---|---|
| Dilute and Shoot (D&S) | Simple dilution of sample with water or buffer [84] | Urine; exploratory analysis when sensitivity is not critical [84] | Fast, cheap, simple; minimal method development [84] | Poor sensitivity; does not remove matrix interferents; can foul instrumentation [84] |
| Protein Precipitation (PPT) | Adding organic solvent (e.g., acetonitrile) to precipitate proteins [84] | Plasma, serum, other protein-rich fluids [84] | Rapid, simple, high-throughput capability in 96-well format [84] | Only removes proteins; leaves phospholipids and other interferents [84] |
| Phospholipid Depletion (PLD) | PPT followed by removal of phospholipids using a scavenging adsorbent [84] | Blood-based samples for LC-MS/MS; reducing ion suppression [84] | Effectively removes a major source of matrix effects in MS [84] | Adds a step to the PPT workflow; may not be needed for all analyses [84] |
| Supported Liquid Extraction (SLE) | Liquid-liquid extraction on a solid support; partitions analytes based on solubility [84] | Targeted extraction of specific analytes from various biofluids [84] | Higher recovery and cleaner extracts than LLE; easier to automate; avoids emulsions [84] | Requires method development; more expensive than scavenging techniques [84] |
Protocol: Protein Precipitation for Plasma/Serum
- Precipitate Proteins: Add a volume of ice-cold acetonitrile (typically 2-3 times the sample volume) to the biofluid sample (e.g., 100 µL plasma). Vortex mix vigorously [84].
- Pellet Precipitate: Centrifuge the mixture at high speed (e.g., 10,000 x g) for 10 minutes to pellet the precipitated proteins [84].
- Recover Supernatant: Carefully transfer the clear supernatant containing the extracted analytes to a new tube [84].
- Concentrate (Optional): The supernatant can be evaporated to dryness under a stream of nitrogen or by vacuum centrifugation. The residue is then reconstituted in a solvent compatible with downstream electrophoresis (e.g., SDS sample buffer) [84].
Workflow Visualization
The following diagram illustrates the overarching decision-making pathway and experimental workflow for preparing different sample types for denaturing gel electrophoresis.
The Scientist's Toolkit: Key Research Reagent Solutions
The following table lists essential reagents and materials required for the sample preparation protocols described in this document.
Table 3: Essential Reagents and Materials for Sample Preparation
| Reagent/Material | Function | Example Product (Thermo Fisher Scientific) |
|---|---|---|
| RIPA Lysis Buffer | A strong, versatile lysis buffer effective for extracting total protein, including membrane-bound and nuclear proteins [36]. | RIPA Lysis Buffer |
| M-PER / T-PER Reagent | Mild, non-denaturing extraction reagents for mammalian cells (M-PER) or tissues (T-PER) that help preserve protein-protein interactions [36]. | M-PER Mammalian Protein Extraction Reagent; T-PER Tissue Protein Extraction Reagent |
| Protease/Phosphatase Inhibitor Cocktail | Added to lysis buffers to prevent proteolysis and maintain protein phosphorylation states by inhibiting endogenous enzymes [36]. | Halt Protease and Phosphatase Inhibitor Cocktail |
| LDS Sample Buffer (4X) | Loading buffer containing lithium dodecyl sulfate (LDS) for denaturing and preparing protein samples for gel electrophoresis [36]. | LDS Sample Buffer (4X) (Cat. No. NP0007) |
| Sample Reducing Agent (10X) | Reduces disulfide bonds in proteins to ensure they are in their linear form for accurate molecular weight separation [36]. | NuPAGE Sample Reducing Agent (10X) |
| BCA Protein Assay Kit | A colorimetric assay for determining protein concentration; compatible with detergents and more uniform across different proteins than Bradford assays [36]. | Pierce BCA Protein Assay Kit (Cat. No. 23225) |
The reliability of any western blot experiment is fundamentally determined long before the first antibody is applied. Successful immunodetection hinges almost entirely on the initial steps of sample preparation, a phase where improper technique can introduce artifacts, mask true results, or lead to complete experimental failure. Within the context of denaturing protein gel electrophoresis research, meticulous sample preparation is not merely a preliminary step but the cornerstone of data integrity. This process ensures that the protein of interest is solubilized, denatured, reduced, and presented to the gel in a state that allows for accurate separation by molecular weight, thereby enabling meaningful detection and analysis downstream. This application note provides a detailed protocol and critical troubleshooting guide to ensure protein samples are optimally prepared for the journey from gel to blot.
Critical Success Factors in Sample Preparation
The transition from a complex cellular environment to a well-defined protein band on a membrane involves several non-negotiable parameters. Attention to the following factors is essential for preserving protein integrity and functionality for immunodetection.
Lysis Buffer Selection and Additives
The choice of lysis buffer is dictated by the subcellular location of the target protein and the nature of the antibody's epitope. For denaturing SDS-PAGE, harsh detergents are required to solubilize proteins fully.
- Buffer Formulations: Radioimmunoprecipitation assay (RIPA) buffer is well-suited for preparing whole-cell extracts, membrane-bound extracts, and nuclear extracts, as it effectively disrupts protein-protein interactions [53]. For cytoplasmic extracts, NP-40 or Triton X-100-containing buffers may be sufficient [53].
- Inhibitor Cocktails: The moment of cell lysis releases proteases and phosphatases. To prevent rapid protein degradation or unwanted post-translational modifications, lysis must be performed on ice with the addition of protease and phosphatase inhibitor cocktails immediately before use [53] [32]. Common inhibitors and their targets are detailed in Table 1.
Table 1: Common Protease Inhibitors for Lysis Buffers
| Inhibitor | Final Concentration | Primary Targets |
|---|---|---|
| PMSF | 1 mM | Serine proteases |
| Aprotinin | 2 µg/mL | Trypsin, Chymotrypsin, Plasmin |
| Leupeptin | 1-10 µg/mL | Lysosomal proteases |
| Pepstatin A | 1 µg/mL | Aspartic proteases |
| EDTA | 1-10 mM | Mg²⁺ and Mn²⁺ metalloproteases |
Reduction and Denaturation
For denaturing SDS-PAGE, proteins must be unfolded and their disulfide bonds broken to ensure migration is proportional to molecular weight.
- Reducing Agents: Disulfide bonds are reduced using agents like dithiothreitol (DTT) at a 50 mM final concentration or β-mercaptoethanol at 2.5% [9]. Freshness is critical; reduced samples should be used immediately or stored for less than an hour before loading to prevent re-oxidation, which produces inconsistent results [9].
- Denaturation Temperature: While boiling at 100°C was once standard, it is now known to promote proteolysis [9]. For optimal results, heat samples for denaturing electrophoresis at 85°C for 2–5 minutes [9]. Do not heat samples for nondenaturing electrophoresis.
Sample Purity and Complexity
Common contaminants in cell lysates can severely disrupt electrophoresis.
- Genomic DNA: Viscosity from genomic DNA affects protein migration and resolution. Shear DNA by sonication, passing the lysate through a narrow-gauge needle, or treating with an endonuclease [9] [53].
- Insoluble Material: Cell lysates contain soluble and insoluble fractions. Centrifuge lysates and load the soluble fraction separately to avoid altered migration patterns [9].
- High Salt and Chaotropes: High salt concentrations increase conductivity, leading to distorted migration and gel artifacts [9] [85]. Similarly, guanidine-HCl precipitates in the presence of SDS. Dialyze samples or use desalting columns to remove these contaminants prior to electrophoresis [9].
Experimental Protocols
Standard Protocol for Preparing Adherent Cell Lysates
This protocol is optimized for denaturing SDS-PAGE followed by western blotting.
Materials:
- Ice-cold Phosphate-Buffered Saline (PBS)
- Appropriate ice-cold lysis buffer (e.g., RIPA buffer) supplemented with fresh protease inhibitors
- 2X or 4X Laemmli sample buffer [53] [86]
- Fresh reducing agent (e.g., 1M DTT)
- Cell scraper
- Microcentrifuge tubes
- Heating block or water bath
Method:
- Cell Washing: Place the culture dish on ice. Aspirate the medium and wash the adherent cells gently with ice-cold PBS [21].
- Lysis: Aspirate the PBS. Add ice-cold lysis buffer (e.g., 1 mL per 10⁷ cells/100 mm dish) [21]. Detach cells using a cell scraper and transfer the cell suspension to a pre-cooled microcentrifuge tube.
- Clarification: Incubate the lysate on ice for 10-30 minutes, then centrifuge at ≥12,000 × g for 10 minutes at 4°C to pellet insoluble debris [21].
- Supernatant Transfer: Transfer the clarified supernatant to a new tube on ice. Discard the pellet.
- Protein Quantification: Determine protein concentration using a compatible assay (e.g., BCA assay) [53].
- Sample Buffer Addition: Dilute an aliquot of the lysate with an equal volume of 2X Laemmli sample buffer. Add fresh DTT to a final concentration of 50 mM [9].
- Denaturation: Heat the samples at 85°C for 2-5 minutes [9].
- Brief Centrifugation: Pulse-centrifuge the samples to collect condensation before loading onto the gel. Samples can be stored at -80°C for future use.
Troubleshooting Common Sample Preparation Issues
Even with careful preparation, issues can arise. Table 2 outlines common problems and their evidence-based solutions.
Table 2: Troubleshooting Sample Preparation for Electrophoresis
| Problem | Potential Cause | Recommended Solution |
|---|---|---|
| Protein Smearing | Sample degradation by proteases. | Use fresh protease inhibitors; keep samples on ice [32] [64]. |
| Incomplete denaturation. | Ensure fresh reducing agent is used; heat at 85°C, not 100°C [9] [85]. | |
| Vertical Streaking | High salt concentration in sample. | Desalt sample via dialysis, precipitation, or desalting column [9] [85]. |
| Bands Clumping in Wells | Protein aggregation/precipitation. | Ensure proper homogenization; add DTT/BME to lysis solution; sonicate sample [87]. |
| No Bands or Faint Bands | Over-reduction of proteins. | Avoid excess reducing agent (e.g., BME, DTT) as it can cause charge repulsion [85]. |
| "Smiling" or "Frowning" Bands | Uneven heating during electrophoresis. | Run gel at lower voltage; ensure buffer levels are even across the gel [64]. |
The Scientist's Toolkit: Essential Reagents
Table 3: Key Research Reagent Solutions for Western Blot Sample Preparation
| Item | Function | Example & Notes |
|---|---|---|
| Lysis Buffer (RIPA) | Solubilizes proteins, disrupts membranes. | Ideal for whole cell, nuclear, and membrane extracts [53]. |
| Protease Inhibitor Cocktail | Prevents protein degradation during and after lysis. | Must be added fresh to lysis buffer [53] [21]. |
| Dithiothreitol (DTT) | Reduces disulfide bonds. | Use at 50 mM final concentration; prepare fresh [9]. |
| Laemmli Sample Buffer | Denatures proteins, provides density for gel loading. | Contains SDS, glycerol, and a tracking dye [53] [86]. |
| BCA Assay Kit | Accurately quantifies total protein concentration. | Compatible with detergents and denaturing reagents [53]. |
Workflow Visualization
The following diagram illustrates the complete pathway from cultured cells to a prepared sample ready for gel loading, integrating the critical steps and decision points outlined in this document.
Troubleshooting Discrepancies Between Expected and Observed Molecular Weights
In denaturing protein gel electrophoresis research, the observation that a protein's apparent molecular weight (MW) differs from its theoretical value is a common challenge. This discrepancy can lead to misinterpretation of data, incorrect protein identification, and flawed experimental conclusions. This application note details the primary causes for these differences and provides validated protocols to identify, troubleshoot, and resolve them, ensuring accurate analysis in drug development and basic research.
Core Reasons for Molecular Weight Discrepancies
The following table summarizes the principal biological and technical factors that cause differences between expected and observed molecular weights on denaturing gels.
Table 1: Common Causes of Molecular Weight Discrepancies and Their Characteristics
| Category | Specific Cause | Effect on Observed MW | Key Characteristics |
|---|---|---|---|
| Protein Processing | Cleavage of Signal/Pro-peptides [88] | Lower than expected | Common for secreted and mitochondrial proteins; results in a mature, shorter protein. |
| Proteolytic Degradation [88] | Lower than expected; smeared or multiple bands | Spurious bands or smears; can be minimized with protease inhibitors. | |
| Post-Translational Modifications (PTMs) | Glycosylation [88] | Significantly higher than expected | Broad or diffuse bands due to heterogeneous sugar chain addition. |
| Phosphorylation [88] | Slightly higher (≈1 kDa per group) | May cause small upward shifts or band splitting; often transient. | |
| Ubiquitination [88] | Higher, with laddering pattern | Can produce a characteristic ladder of bands, each increasing by ~8 kDa (ubiquitin). | |
| Protein Structure & Complexes | Non-covalent Complexes [88] | Higher than monomeric MW | Persistent homo- or hetero-dimers/multimers despite denaturing conditions. |
| Protein Aggregation [89] | Much higher than expected | Slower migration due to dimers or larger aggregates from sample prep conditions. | |
| Alternative Splicing & Isoforms [88] | Higher or lower, multiple bands | Presence of distinct bands representing different protein variants from the same gene. | |
| Technical & Experimental Factors | Antibody Cross-reactivity [88] | Unpredictable, non-specific bands | Bands that do not correspond to known isoforms or modifications. |
| Incomplete Denaturation/Reduction [89] | Higher than expected | Residual disulfide bonds can cause aggregation; remedied with DTT/β-mercaptoethanol. | |
| Gel/Buffer System [90] | Altered mobility | Prestained markers migrate differently in Tris-Glycine vs. Bis-Tris gels. |
Detailed Experimental Protocols for Identification and Resolution
Protocol: Validating Protein Identity and PTMs
This protocol is designed to confirm whether an observed band is the target protein and to investigate the potential role of PTMs.
I. Materials
- Research Reagent Solutions:
- Lysis Buffer: RIPA buffer supplemented with 1X protease and phosphatase inhibitors.
- SDS-PAGE Sample Buffer (2X): 100 mM Tris-HCl (pH 6.8), 4% SDS, 20% glycerol, 0.2% Bromophenol Blue.
- Reducing Agent: 500 mM Dithiothreitol (DTT) or 20% β-mercaptoethanol.
- Enzymes: PNGase F (for N-linked deglycosylation), Endo H (for high-mannose N-glycans), Lambda Protein Phosphatase.
- Cell Lines: Positive control (overexpressing the target protein, preferably tagged) and negative control (KO/Knockdown cell line).
II. Method
- Sample Preparation:
- Lyse cells or tissues in ice-cold lysis buffer. Centrifuge at 12,000 x g for 15 minutes at 4°C to remove insolubles.
- Determine protein concentration using a compatible assay (e.g., BCA).
- Prepare samples by mixing equal volumes of protein lysate and 2X SDS-PAGE Sample Buffer. Add DTT to a final concentration of 50-100 mM.
- Denature samples by heating at 95°C for 5-10 minutes. Incomplete heating is a major cause of aggregation and higher MW appearance [89].
Enzymatic Treatment for PTM Investigation:
- For Glycosylation: Denature 20-30 µg of protein in 1X Glycoprotein Denaturing Buffer at 95°C for 10 minutes. Cool, then add NP-40, G7 Reaction Buffer, and PNGase F. Incubate at 37°C for 1-3 hours.
- For Phosphorylation: Incubate 20-30 µg of protein with Lambda Protein Phosphatase and supplied buffer at 30°C for 30-60 minutes.
- After enzymatic treatment, add standard SDS-PAGE sample buffer and proceed with electrophoresis.
Electrophoresis and Western Blotting:
- Load samples, including pre-stained protein markers, positive controls, and negative controls.
- Run SDS-PAGE at constant voltage (recommended 80-120V) until the dye front migrates to the bottom.
- Transfer to PVDF or nitrocellulose membrane.
- Probe with a validated primary antibody against your target protein and an appropriate HRP-conjugated secondary antibody.
- Develop with enhanced chemiluminescence (ECL) reagent and image.
III. Data Interpretation
- A collapse to a lower MW after PNGase F treatment confirms N-linked glycosylation.
- A downward shift after phosphatase treatment suggests phosphorylation.
- Bands present in the positive control but absent in the negative control are specific to the target protein.
Protocol: Optimizing Sample Preparation to Minimize Degradation and Aggregation
This protocol addresses technical artifacts like proteolysis and aggregation.
I. Materials
- Research Reagent Solutions:
- Protease-Inhibited Lysis Buffer: As in Protocol 2.1.
- Fresh Reducing Agent: Always prepare DTT fresh or use from a recently opened, aliquoted stock.
- Strong Denaturant Buffer: 8 M Urea or 6 M Guanidine-HCl in lysis buffer.
II. Method
- Prevent Proteolysis:
- Perform all steps on ice or at 4°C.
- Include a broad-spectrum protease inhibitor cocktail in all lysis and storage buffers.
- Avoid repeated freeze-thaw cycles of protein lysates. Store aliquots at -80°C.
Ensure Complete Denaturation and Reduction:
- For proteins suspected of forming stable complexes or aggregates, use a stronger denaturant buffer (e.g., with 8 M Urea) during initial solubilization.
- Increase the concentration of reducing agent. If using 50 mM DTT, try 100 mM. Alternatively, incubate with reducing agent for 10-15 minutes at room temperature before heating.
- For stubborn aggregates, a brief sonication of the sample after heating can help.
Troubleshoot Gel Electrophoresis Conditions:
- Ensure the gel percentage is appropriate for your protein's MW. Use lower percentage gels (e.g., 8-10%) for high MW proteins and higher percentages (e.g., 12-15%) for lower MW proteins [63].
- Verify that the electrodes are connected correctly. Reversed polarity will cause proteins to run out of the gel [63].
- Avoid overloading the gel, as this can cause smearing and poor resolution. Load 0.1–0.2 μg of protein per millimeter of gel well width [63].
Validation and Advanced Techniques
When SDS-PAGE results are ambiguous, orthogonal techniques are required for validation.
- Mass Spectrometry (MS): This is the gold standard for determining exact molecular weight and identifying PTMs. Software tools like Census can process MS data for quantitative analysis, helping to characterize protein samples with high accuracy [91].
- Alternative Molecular Weight Determination: Techniques like Multi-Angle Light Scattering (MALS) coupled with Size-Exclusion Chromatography (SEC) provide absolute molecular weight values without relying on calibration standards, eliminating uncertainties associated with gel migration [92].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Troubleshooting Molecular Weight Discrepancies
| Reagent | Function | Example Use Case |
|---|---|---|
| DTT (Dithiothreitol) | Reducing agent; breaks disulfide bonds. | Prevents protein aggregation by reducing intermolecular disulfide bonds [89]. |
| Protease Inhibitor Cocktail | Inhibits serine, cysteine, metallo-proteases, etc. | Prevents non-specific proteolytic cleavage during sample preparation [88]. |
| Phosphatase Inhibitors | Preserves protein phosphorylation state. | Added to lysis buffer when studying phosphoproteins to prevent dephosphorylation. |
| PNGase F | Enzyme that removes N-linked glycans. | Confirms N-linked glycosylation by a downward MW shift on a gel [88]. |
| SDS (Sodium Dodecyl Sulfate) | Ionic detergent; denatures proteins. | Coats proteins with a uniform negative charge, allowing separation primarily by size. |
| Positive Control Lysate | Lysate from cells overexpressing the target protein. | Validates antibody specificity and serves as a reference for expected bands [88]. |
| Tag-Specific Antibodies | Antibodies against epitope tags (e.g., GFP, HA). | Used with tagged protein overexpression to confirm the identity of the target band [88]. |
Visual Workflows for Troubleshooting
The following diagram illustrates the systematic decision-making process for diagnosing and resolving molecular weight discrepancies.
Diagram 1: A systematic workflow for diagnosing molecular weight discrepancies.
The second diagram illustrates the core molecular mechanisms that lead to changes in a protein's apparent molecular weight.
Diagram 2: Molecular mechanisms behind MW changes.
Discrepancies between theoretical and observed molecular weights are multifactorial, arising from authentic biological processing or technical artifacts. By employing the systematic troubleshooting workflows, detailed protocols, and validation strategies outlined in this document, researchers can accurately interpret their Western blot and electrophoresis data, thereby strengthening the foundation of their protein research and drug development efforts.
Best Practices for Documenting and Reproducing Your Sample Preparation Workflow
Within the broader context of denaturing protein gel electrophoresis research, the sample preparation workflow is a critical foundation upon which reliable and reproducible results are built. Proper documentation of this process is not merely an administrative task; it is a fundamental scientific practice that ensures the integrity of your data, enables the replication of your experiments by your future self and other researchers, and provides clarity on the precise conditions that led to your findings. This application note provides detailed protocols and best practices for documenting your sample preparation to achieve the highest standards of reproducibility, specifically tailored for researchers, scientists, and drug development professionals working with protein samples.
Core Principles of Sample Denaturation for SDS-PAGE
The primary goal of sample preparation for denaturing polyacrylamide gel electrophoresis (SDS-PAGE) is to dismantle the native structure of proteins, rendering them into linear polypeptides whose migration will depend primarily on molecular weight [17]. A protein's functional, three-dimensional structure is maintained by several forces, including hydrogen bonding, hydrophobic interactions, Van der Waal's forces, and disulfide bonding [17]. Effective denaturation must disrupt all these interactions.
Tertiary and quaternary structures, which involve the three-dimensional folding of a single polypeptide chain and the interaction of multiple polypeptide chains, respectively, are key targets. While SDS and heat are sufficient to break non-covalent bonds, the covalent disulfide bonds between cysteine residues require a strong reducing agent for disruption [17]. Failure to completely denature the sample can result in anomalous band migration, smearing, and unreliable molecular weight estimation.
Experimental Protocol: Denaturing Sample Preparation
Reagent Preparation: 2× Concentrated Sample Buffer
The following formulation provides robust denaturation for a wide range of protein samples. Prepare a 2× concentrate for convenient use.
Table 1: Composition of 2X Denaturing Sample Buffer
| Component | Final Concentration in Prepared Sample | Function and Rationale |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) | 1% | A strong anionic detergent that binds to polypeptide chains, imparting a uniform negative charge and disrupting secondary and tertiary structure by breaking hydrogen bonds and neutralizing protein charges [17]. |
| DTT (Dithiothreitol) | 80 mM | A reducing agent that cleaves disulfide bonds between cysteine residues, thereby disrupting tertiary and quaternary structure. Preferred over 2-mercaptoethanol due to its lower odor and often superior efficacy [17]. |
| Tris-Cl, pH 6.8 | 10 mM | Provides a buffered environment at the specific pH required for the stacking process in discontinuous gel electrophoresis [17]. |
| Glycerol | 10% | Increases the density of the sample solution, ensuring it settles neatly at the bottom of the sample well and does not float out when loaded onto the gel [17]. |
| EDTA (Ethylenediaminetetraacetic acid) | 1 mM | A chelating agent that binds divalent cations (e.g., Ca²⁺, Mg²⁺). This reduces the activity of metal-dependent proteolytic enzymes, thereby protecting your sample from degradation [17]. |
| Bromophenol Blue | ~0.05 mg/ml | A tracking dye that migrates at the leading edge of the protein front, allowing visual monitoring of electrophoresis progress [17]. |
Step-by-Step Sample Preparation Protocol
- Protein Concentration Determination: Determine the protein concentration of your crude sample using a standard assay (e.g., BCA, Bradford).
- Sample Dilution: Dilute all protein samples to a concentration of 2 mg/mL using an appropriate buffer or purified water. The final volume prepared should be at least double the volume required to load your gel.
- Buffer Mixing: Mix 1 volume of the diluted protein sample (2 mg/mL) with 1 volume of the 2× concentrated sample buffer. This yields a final protein concentration of 1 mg/mL in 1× sample buffer.
- Denaturation: Heat the sample-buffer mixture in a steaming water bath or heating block for at least 10 minutes. For membrane-associated proteins, more vigorous heating may be necessary.
- Cooling and Storage: Briefly centrifuge samples to collect condensation. Denatured samples can be stored at room temperature for immediate use or frozen at -20°C for future analysis.
Workflow Visualization
The following diagram summarizes the logical workflow for preparing samples for denaturing gel electrophoresis, ensuring consistency and reproducibility.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagent Solutions for Sample Denaturation
| Item | Category | Function / Application Notes |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) | Denaturant | Unfolds proteins and confers negative charge. Critical for masking intrinsic protein charge. |
| DTT (Dithiothreitol) | Reducing Agent | Reduces disulfide bonds. More stable and less pungent than 2-mercaptoethanol. |
| Tris-HCl Buffer | Buffer | Maintains optimal pH (6.8) for stacking in Laemmli-style SDS-PAGE systems. |
| Protease Inhibitor Cocktails | Additive | Protects protein samples from proteolytic degradation during preparation. |
| EDTA | Chelating Agent | Inhibits metalloproteases by chelating divalent cations like Ca²⁺ and Mg²⁺. |
| Glycerol | Density Agent | Adds density to sample for easy gel loading. |
| Bromophenol Blue | Tracking Dye | Visual marker for electrophoresis progress. |
Data Presentation and Documentation Standards
Precise documentation in the Materials and Methods section is crucial for reproducibility. Report the general procedure without exhaustive volume calculations for every sample, which is considered amateurish [17].
Best Practices for Reporting:
- State the final concentrations of all critical components in the prepared sample (e.g., "Samples were denatured in a buffer containing 1% SDS, 10% glycerol, 80 mM DTT, and 0.05 mg/mL bromophenol blue in 10 mM Tris-Cl, pH 6.8.").
- Specify the denaturation conditions precisely, including temperature and duration (e.g., "Samples were heated at 95°C for 10 minutes.").
- Note the protein load per gel lane (e.g., "20 µg of total protein was loaded per lane."). A rule of thumb for mini-slab gels is to load about 0.5 µg protein per expected band [17].
Troubleshooting and Optimization
Even with a standardized protocol, optimization may be required for specific sample types.
Table 3: Troubleshooting Common Sample Preparation Issues
| Observation | Potential Cause | Recommended Solution |
|---|---|---|
| Smearing or Streaking | Protein aggregation due to overloading or insufficient denaturation. | Reduce the amount of protein loaded. Ensure fresh reducing agent (DTT) is used and heating is sufficient. |
| Incomplete Denaturation | Insufficient heating or inactive reducing agent. | Increase heating time or temperature. Prepare a fresh aliquot of DTT. |
| Poor Resolution | Proteolytic degradation. | Include protease inhibitors during sample preparation and keep samples on ice prior to denaturation. |
| Inconsistent Replicates | Variation in sample buffer mixing or heating. | Ensure consistent volumes and vortexing before heating. Use a calibrated heating block. |
Conclusion
Mastering sample preparation is the non-negotiable foundation for successful denaturing protein gel electrophoresis. By understanding the core principles, executing meticulous protocols, systematically troubleshooting artifacts, and rigorously validating results, researchers can generate highly reproducible and reliable data. This comprehensive approach directly enhances the integrity of downstream applications, from western blotting to proteomic analysis, ultimately accelerating discoveries in biomedical research, drug development, and clinical diagnostics. Future directions will likely involve further standardization of protocols and the integration of automated sample preparation systems to enhance throughput and consistency across laboratories.
References
- 1. Protein Electrophoresis Using SDS-PAGE: A Detailed ... [https://www.goldbio.com/blogs/articles/protein-electrophoresis-sds-page-overview?srsltid=AfmBOoqd1J5tKLy25BsQxPcdv9wY7n6Mv5mNWcz3xNX7jZmkFzT6yQ7O]
- 2. The Evolution of SDS-Based Protein Separation [https://www.bio-techne.com/resources/blogs/advantages-of-ce-sds]
- 3. SDS-PAGE [https://en.wikipedia.org/wiki/SDS-PAGE]
- 4. SDS-PAGE & Protein Separation Role of SDS Explained [https://www.letstalkacademy.com/sds-page-protein-separation-role-of-sds-explained/]
- 5. Deciphering food proteins: The applications of SDS-PAGE ... [https://www.sciencedirect.com/science/article/abs/pii/S2212429225003025]
- 6. SDS-PAGE Electrophoresis Market Size & Share 2025-2032 [https://www.360iresearch.com/library/intelligence/sds-page-electrophoresis]
- 7. SDS-PAGE Electrophoresis Market - Global Forecast 2025- ... [https://www.researchandmarkets.com/reports/6140756/sds-page-electrophoresis-market-global?srsltid=AfmBOoo1WCxS5dZa4RDg6bYRbhlJYpHmNOxe79wCkK06ftqbbrMCBH2E]
- 8. SDS-PAGE: The science behind all those bubbles [https://www.antibodiesinc.com/pages/sds-page-demystified?srsltid=AfmBOooNbPP9_l4HpXAr3gxsltkZ7-pXR7mtRhRYjSQSfUj5nKh0Y4xk]
- 9. Gel Electrophoresis Sample Preparation [https://www.thermofisher.com/us/en/home/references/protein-analysis-guide/protein-separation/gel-electrophoresis-sample-preparation.html]
- 10. The Nature of Denaturing (Protein Gels, that is!) [https://bitesizebio.com/20794/the-nature-of-denaturing-protein-gels-that-is/]
- 11. SDS-Page - Ask A Biologist [https://askabiologist.asu.edu/sds-page]
- 12. The principle and method of polyacrylamide gel ... [https://www.mblbio.com/bio/g/support/method/sds-page.html]
- 13. SDS-PAGE: The science behind all those bubbles [https://www.antibodiesinc.com/pages/sds-page-demystified?srsltid=AfmBOoo311-Qpu1e2Xrl-r9lKyNAkEqYgU-ac7ZkdwQjMgvbRya4Bsya]
- 14. How SDS-PAGE Works [https://bitesizebio.com/580/how-sds-page-works/]
- 15. SDS-PAGE - Mullins Lab - [https://mullinslab.ucsf.edu/sds-page/]
- 16. Native SDS-PAGE: High Resolution Electrophoretic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4517606/]
- 17. Preparing protein samples for sds-page [https://www.ruf.rice.edu/~bioslabs/studies/sds-page/denature.html]
- 18. SDS-PAGE guide: Sample preparation to analysis [https://www.abcam.com/en-us/knowledge-center/western-blot/sds-page-guide]
- 19. Laemmli Buffer: The 5 Critical Components [https://bitesizebio.com/44540/laemmli-buffer-what-is-it-for-anyway/]
- 20. One-Dimensional SDS and Non-Denaturing Gel ... [https://www.thermofisher.com/us/en/home/references/protocols/proteins-expression-isolation-and-analysis/sds-page-protocol/one-dimensional-sds-and-non-denaturing-gel-electrophoresis.html]
- 21. Western blotting guide: Part 1, Introduction and Sample ... [https://www.jacksonimmuno.com/secondary-antibody-resource/technical-tips/western-blotting-guide-part-1/]
- 22. SDS PAGE [https://www.bostonbioproducts.com/research-and-discovery/sds-page/]
- 23. Overview of Protein Electrophoresis [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-electrophoresis.html]
- 24. Differences between SDS PAGE and Native PAGE [https://www.geeksforgeeks.org/biology/differences-between-sds-page-and-native-page/]
- 25. Native Polyacrylamide Gels [https://link.springer.com/protocol/10.1007/978-1-4939-8793-1_8]
- 26. Native Versus Denaturing Gels [https://bitesizebio.com/27332/native-versus-denaturing-gels/]
- 27. Denaturing Gel Electrophoresis - an overview [https://www.sciencedirect.com/topics/chemistry/denaturing-gel-electrophoresis]
- 28. How current, voltage, and power settings affect SDS-PAGE [https://www.biossusa.com/blogs/news/how-current-voltage-and-power-settings-affect-sds-page?srsltid=AfmBOoqBJ2ZvtbTc88dOlQiZP5hlVxQSQ7gkB5claW4mrWYlgnkg3yOA]
- 29. Gel Electrophoresis Steps [https://azurebiosystems.com/blog/gel-electrophoresis-steps/]
- 30. Highlights from FDA's Analytical Test Method Validation ... [https://www.propharmagroup.com/thought-leadership/fda-analytical-test-method-validation-guidance]
- 31. Analysis of RNA Yield, Integrity, and Purity [https://www.biopharminternational.com/view/validating-rna-quantity-and-quality-analysis-rna-yield-integrity-and-purity]
- 32. Preparing High-Quality Samples for Electrophoresis: Best ... [https://www.labx.com/resources/preparing-high-quality-samples-for-electrophoresis-best-practices/5550]
- 33. Image integrity and standards | Nature Portfolio [https://www.nature.com/nature-portfolio/editorial-policies/image-integrity]
- 34. RNA quantification and quality control methods [https://www.qiagen.com/us/knowledge-and-support/knowledge-hub/bench-guide/rna/rna-analysis/quantification-and-analysis-of-rna]
- 35. Sample preparation for western blot [https://www.abcam.com/en-us/technical-resources/guides/western-blot-guide/sample-preparation-for-western-blot]
- 36. Western Blot Sample Preparation Protocol [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-gel-electrophoresis-information/western-blot-protocols/western-blot-sample-preparation.html]
- 37. Comparing Efficiency of Lysis Buffer Solutions and Sample ... [https://www.mdpi.com/1420-3049/27/11/3390]
- 38. Protein Extraction Methods Suitable for Muscle Tissue ... [https://www.mdpi.com/2227-7382/12/4/27]
- 39. High-Efficiency Protein Extraction From Lupine Roots [https://pmc.ncbi.nlm.nih.gov/articles/PMC12514137/]
- 40. Western blot protocol [https://www.abcam.com/en-us/technical-resources/protocols/western-blot]
- 41. Dithiothreitol (DTT) Common Applications [https://agscientific.com/blog/dithiothreitol-dtt-applications.html?srsltid=AfmBOooljEYgRjVbVwfyM_s846EIL7sw_mIAMKOx0jZ5McPhvAKVnT55]
- 42. Blog - PotM: TCEP as Versatile Reducing Agent [https://iris-biotech.de/global/blog/potm-tris-2-carboxyethyl-phosphine-tcep-as-versatile-reducing-agent.html]
- 43. Protein Denaturing and Reducing Agents [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-labeling-crosslinking/protein-modification/reducing-agents-protein-disulfides.html]
- 44. Dithiothreitol - an overview [https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/dithiothreitol]
- 45. Bradford protein assay [https://www.abcam.com/en-us/knowledge-center/western-blot/bradford-assay]
- 46. Differences in Protein Assays and Their Results [https://absbio.com/white-papers/differences-in-protein-assays-and-their-results/]
- 47. The Difference Between the BCA and Bradford Protein Assays [https://info.gbiosciences.com/blog/the-difference-between-the-bca-and-bradford-protein-assays]
- 48. Chemistry of Protein Assays | Thermo Fisher Scientific - TW [https://www.thermofisher.com/tw/zt/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/chemistry-protein-assays.html]
- 49. : Which to Choose? Bradford vs BCA Assay [https://opentrons.com/applications/bradford-assay/bradford-vs-bca]
- 50. Bradford Protein Assay [https://bio-protocol.org/exchange/protocoldetail?id=45&type=1]
- 51. Pierce BCA Protein Assay Protocol [https://www.protocols.io/view/pierce-bca-protein-assay-protocol-dyti7wke.html]
- 52. Protocol for Bradford Protein Assay [https://www.creative-proteomics.com/resource/protocol-for-bradford-protein-assay.htm]
- 53. Western Blot: The Complete Guide [https://www.antibodies.com/applications/western-blotting]
- 54. SDS-PAGE in Food Science: Elevating Protein Insight and ... [https://www.eurofinsus.com/food-testing/resources/sds-page-in-food-science-elevating-protein-insight-and-product-integrity/]
- 55. Comparing SDS-PAGE and CE-SDS for Antibody Purity ... [https://www.bioprocessintl.com/qa-qc/comparing-sds-page-and-ce-sds-for-antibody-purity-analysis]
- 56. SDS-PAGE Protocol & Troubleshooting - Creative Biolabs [https://www.creativebiolabs.net/sds-page.htm]
- 57. How does Protein Concentration affect SDS PAGE results in ... [https://kendricklabsinc.wordpress.com/2025/09/15/how-does-protein-concentration-affect-sds-page-results-in-research/]
- 58. Common artifacts and mistakes made in electrophoresis [https://pmc.ncbi.nlm.nih.gov/articles/PMC7295095/]
- 59. Protein Denaturation - an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/protein-denaturation]
- 60. Denaturation (biochemistry) [https://en.wikipedia.org/wiki/Denaturation_(biochemistry)]
- 61. Smeared protein gels [https://www.ruf.rice.edu/~bioslabs/studies/sds-page/gelgoofs/smear.html]
- 62. Smeared Bands [https://www.nationaldiagnostics.com/2013/08/28/smeared-bands/]
- 63. Nucleic Acid Gel Electrophoresis Troubleshooting [https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-learning-center/invitrogen-school-of-molecular-biology/na-electrophoresis-education/na-electrophoresis-troubleshooting.html]
- 64. Troubleshooting Common Electrophoresis Problems and ... [https://www.labx.com/resources/troubleshooting-common-electrophoresis-problems-and-artifacts/5539]
- 65. 五种电泳技术的比较参数_北京六一 - 电泳仪 [http://www.liuyi17.com/jishu/303.html]
- 66. An Electric Field and Runtime Driven Band Model for High- ... [https://www.sciencedirect.com/science/article/abs/pii/S0003267025002673]
- 67. Constant Current or Voltage in SDS-PAGE [https://bitesizebio.com/51744/constant-current-or-voltage-in-sds-page/]
- 68. Journal of Analytical & Bioanalytical Techniques [https://www.omicsonline.org/open-access/gel-electrophoresis-an-essential-technique-in-molecular-biology-125744.html]
- 69. Direct observation of fluorescent proteins in gels [https://pmc.ncbi.nlm.nih.gov/articles/PMC11808229/]
- 70. Troubleshooting SDS-PAGE Gel Running Issues [https://www.goldbio.com/blogs/articles/troubleshooting-sds-page-gel-running-issues?srsltid=AfmBOopbBsm2QBcxab6gRd1-KjjWzhYiua4hrV0pgU0ePQEqaQHPdOQH]
- 71. The gel edge electric field gradients in denaturing ... [https://pubmed.ncbi.nlm.nih.gov/9629888/]
- 72. Quantitative analysis of one-dimensional gel ... [https://pubmed.ncbi.nlm.nih.gov/2361188/]
- 73. How to Minimize System Noise in Gel Electrophoresis ... [https://eureka.patsnap.com/report-how-to-minimize-system-noise-in-gel-electrophoresis-output]
- 74. Mastering Electrophoresis: Techniques, Equipment, and ... [https://www.labx.com/resources/mastering-electrophoresis-techniques-equipment-and-applications-in-dna-rna-and-protei/5535]
- 75. DNA Electrophoresis vs Protein Electrophoresis [https://blog.edvotek.com/2025/07/24/dna-electrophoresis-vs-protein-electrophoresis/]
- 76. Optimizing Gel Electrophoresis: Best Practices for Sample ... [https://www.gelepchina.com/news/optimizing-gel-electrophoresis-best-practices-for-sample-volume-voltage-and-timing/]
- 77. Molecular Weight Markers | DNA Ladders [https://www.promega.com/products/cloning-and-dna-markers/dna-ladder-rna-ladder/]
- 78. Molecular-weight size marker [https://en.wikipedia.org/wiki/Molecular-weight_size_marker]
- 79. Protein Standards & Ladders [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-gel-electrophoresis/protein-standards-ladders.html]
- 80. How do I determine the concentration, yield and purity of ... [https://www.promega.com/resources/pubhub/enotes/how-do-i-determine-the-concentration-yield-and-purity-of-a-dna-sample/]
- 81. How should I QC my genomic DNA samples before ... [https://dnatech.ucdavis.edu/faqs/how-should-i-qc-my-genomic-dna-samples-before-sequencing]
- 82. Nucleic Acid Quantification Methods: A Comprehensive ... [https://www.blue-raybio.com/en/blog/Nucleic-Acid-Quantification-Methods-A-Comprehensive-Guide]
- 83. The suitability of using spectrophotometry to determine ... [https://www.sciencedirect.com/science/article/abs/pii/S0889157522003076]
- 84. Sample Preparation for Biofluids [https://www.biotage.com/biofluids-sample-prep]
- 85. Protein Gel 1D Electrophoresis Support—Troubleshooting [https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/protein-electrophoresis-western-blotting-support-center/protein-gel-1d-electrophoresis-support1/protein-gel-1d-electrophoresis-support-troubleshooting.html]
- 86. Western Blot Protocols and Recipes [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-gel-electrophoresis-information/western-blot-protocols.html]
- 87. Troubleshooting SDS-PAGE Sample Preparation Issues [https://www.goldbio.com/blogs/articles/troubleshooting-sds-page-sample-preparation-issues?srsltid=AfmBOopqwLpwNyGYXKg0EJqFKx9SgLa9xHV7AMdLSWjP6FKY_zT2GauF]
- 88. Why Does the Molecular Weight of My Protein Differ From ... [https://www.technologynetworks.com/proteomics/articles/why-does-the-molecular-weight-of-my-protein-differ-from-the-theoretically-expected-weight-322079]
- 89. What Are the Possible Reasons for the Molecular Weight of ... [https://www.mtoz-biolabs.com/what-are-the-possible-reasons-for-the-molecular-weight-of-proteins-detected-by-sds-page-being-higher-than-expected.html]
- 90. FAQ: Why do the apparent molecular weight values for ... [https://www.neb.com/en-us/faqs/2012/02/06/why-do-the-apparent-molecular-weight-values-for-the-prestained-protein-markers-appear-to-be-diffe?srsltid=AfmBOopSgjDdirzcpkpkKEih7T4U0O9P2CEB1z92WracBF_cLJtqP_nr]
- 91. A quantitative analysis software tool for mass spectrometry ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3509211/]
- 92. 3 types of calculated molecular weight data [https://www.malvernpanalytical.com/en/learn/knowledge-center/insights/3-types-calculated-molecular-weight-data]