Optimizing PCR with DMSO and Betaine: A Strategic Guide for Amplifying Challenging Templates
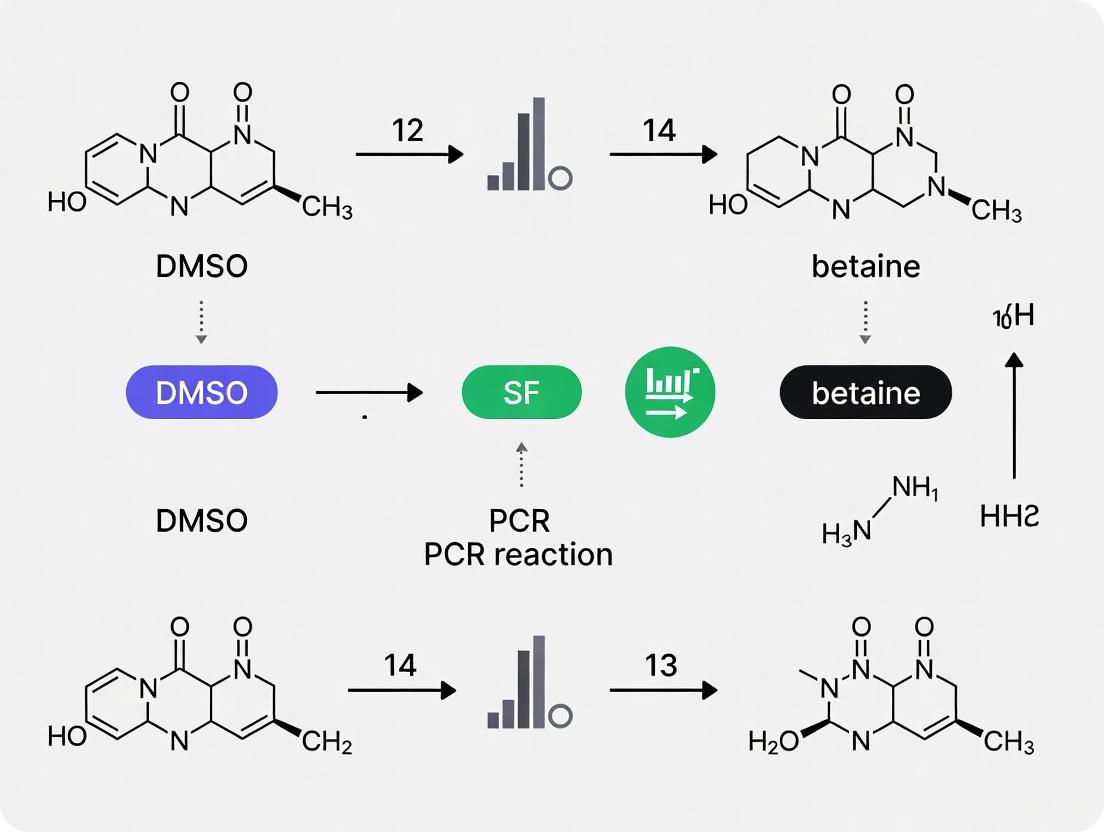
This article provides a comprehensive guide for researchers and drug development professionals on the strategic use of DMSO and betaine to overcome significant challenges in PCR, particularly with GC-rich templates...
Optimizing PCR with DMSO and Betaine: A Strategic Guide for Amplifying Challenging Templates
Abstract
This article provides a comprehensive guide for researchers and drug development professionals on the strategic use of DMSO and betaine to overcome significant challenges in PCR, particularly with GC-rich templates and sequences prone to stable secondary structures. It covers the foundational science behind how these additives work, detailing their distinct mechanisms for facilitating DNA amplification. The content delivers actionable, step-by-step methodological protocols for incorporating DMSO and betaine, either individually or sequentially, into reaction setups. A dedicated troubleshooting section addresses common pitfalls and optimization strategies, while a comparative analysis validates their performance against other enhancers and in demanding applications like DNA barcoding and de novo gene synthesis, empowering scientists to achieve robust and reliable amplification results.
Understanding the Science: How DMSO and Betaine Overcome PCR Barriers
The Challenge of GC-Rich Regions and Secondary Structures in PCR
Troubleshooting Guides
FAQ 1: Why are GC-rich DNA sequences particularly challenging to amplify by standard PCR?
GC-rich DNA sequences (defined as those containing ≥60% guanine and cytosine bases) present several unique challenges for PCR amplification [1] [2]. The primary difficulties stem from the inherent biochemical properties of GC base pairs:
- Enhanced Thermal Stability: G-C base pairs form three hydrogen bonds compared to only two in A-T base pairs, creating significantly more thermostable DNA duplexes that require more energy to denature [1] [2].
- Secondary Structure Formation: GC-rich sequences readily form stable secondary structures such as hairpin loops and stem-loop configurations that can block polymerase progression [1] [3]. These structures are stable even at typical PCR denaturation temperatures (92-95°C).
- Polymerase Stalling: DNA polymerases frequently stall at these complex secondary structures, resulting in shorter, incomplete amplification products [1].
- Non-specific Primer Binding: Primers designed for GC-rich templates tend to form dimers and exhibit mispriming due to the stable interactions [1] [2].
These challenges commonly manifest experimentally as blank gels, DNA smears, multiple non-specific bands, or complete PCR failure [1].
FAQ 2: How do DMSO and betaine work synergistically to improve GC-rich PCR amplification?
DMSO (dimethyl sulfoxide) and betaine function through complementary mechanisms to overcome the challenges of GC-rich DNA amplification. When used in combination, they create a powerful enhancing effect that is greater than either additive used alone [4].
Table 1: Mechanism of Action of DMSO and Betaine in GC-Rich PCR
| Additive | Final Concentration | Primary Mechanism | Effect on PCR |
|---|---|---|---|
| DMSO | 5-10% [5] | Disrupts secondary structures by interfering with hydrogen bonding and base stacking [1] [3] | Reduces formation of stable hairpins and stem-loops |
| Betaine | 0.5M-2.5M [6] [4] | Equalizes the thermal stability of AT and GC base pairs by occupying DNA grooves [4] | Reduces DNA melting temperature, improves strand separation |
The synergistic effect of combining DMSO and betaine was demonstrated in a study attempting to amplify a RET promoter region with 79% GC content [4]. While neither additive alone produced specific amplification, their combination successfully yielded the target amplicon. For particularly challenging templates (>75% GC), adding 7-deaza-dGTP (50μM) to the DMSO/betaine mixture creates a triple-additive system that can overcome even the most refractory amplifications [4].
FAQ 3: What is the optimized experimental protocol for implementing DMSO and betaine in GC-rich PCR?
The following optimized protocol has been successfully used to amplify DNA regions with GC content exceeding 75% [4] [5]:
Reagent Setup (25μL reaction):
- Template DNA: 100ng genomic DNA or 10-50ng of plasmid DNA [7]
- Primers: 10-50pmol each (0.1-1μM final concentration) [7]
- dNTPs: 200μM each dNTP [6] [4]
- PCR Buffer: 1X concentration (as supplied with polymerase)
- MgClâ‚‚: 1.5-2.0mM (optimize from 1.0-4.0mM) [5]
- Taq DNA Polymerase: 1.25 units [4]
- DMSO: 5% final concentration [5]
- Betaine: 1.3M final concentration [4]
- Sterile distilled water: to volume
Thermal Cycling Conditions:
- Initial Denaturation: 94°C for 3-5 minutes
- Amplification (35-40 cycles):
- Denaturation: 94°C for 30 seconds
- Annealing: Temperature gradient 60-69°C for 30 seconds (optimize 7°C above calculated Tm) [5]
- Extension: 72°C for 1 minute per kb
- Final Extension: 72°C for 7 minutes
- Hold: 4°C indefinitely
For extremely challenging templates, incorporate a "slow-down PCR" approach with reduced ramp rates between annealing and extension steps, and consider adding 7-deaza-dGTP (50μM) as a dGTP analog [4] [3].
FAQ 4: What other critical parameters should be optimized when amplifying GC-rich regions?
Beyond additive incorporation, several additional parameters require careful optimization for successful GC-rich PCR:
Polymerase Selection: Standard Taq polymerase often fails with GC-rich templates. Consider specialized enzymes like OneTaq Hot Start DNA Polymerase with GC Buffer or Q5 High-Fidelity DNA Polymerase with GC Enhancer, which are specifically formulated for difficult amplicons [1] [2].
Magnesium Concentration Optimization: Mg²⺠acts as a essential polymerase cofactor, but optimal concentrations vary. Test a gradient from 1.0-4.0mM in 0.5mM increments to find the ideal concentration that balances specificity and yield [1] [2].
Annealing Temperature Optimization: GC-rich templates typically require higher annealing temperatures than calculated. Implement a temperature gradient PCR testing range from 60-72°C, or 7°C above the calculated Tm [5]. Higher temperatures increase primer specificity but may reduce yield, potentially requiring additional PCR cycles [1].
Initial Denaturation Strategy: For the first few cycles, consider increasing denaturation temperature to 95-98°C to help melt stubborn secondary structures, then reduce to standard temperatures for remaining cycles to preserve polymerase activity [3].
Table 2: Optimal Concentration Ranges for PCR Additives in GC-Rich Amplification
| Additive | Working Concentration | Optimal Concentration | Key Considerations |
|---|---|---|---|
| DMSO | 1-10% [6] | 5% [5] | Higher concentrations may inhibit polymerase activity |
| Betaine | 0.5M-2.5M [6] | 1.3M [4] | Equalizes template stability; especially useful >70% GC |
| 7-deaza-dGTP | 50-150μM | 50μM [4] | Use as partial substitute for dGTP (25-50% replacement); may affect downstream applications |
| Glycerol | 1-10% [6] | 5-10% | Reduces secondary structures; typically less effective than DMSO/betaine |
| Formamide | 1.25-10% [6] | 1.25-5% | Increases primer stringency; use when non-specific binding is primary issue |
Experimental Workflow Visualization
GC-Rich PCR Troubleshooting Workflow
Research Reagent Solutions
Table 3: Essential Reagents for GC-Rich PCR Optimization
| Reagent Category | Specific Examples | Function & Application |
|---|---|---|
| Specialized Polymerases | OneTaq GC-rich Enzyme (NEB), Q5 High-Fidelity (NEB), AccuPrime GC-Rich (ThermoFisher) | Formulated with enhanced processivity through stable secondary structures; often include proprietary GC enhancers [1] [3] |
| PCR Additives | DMSO (Sigma), Betaine (Sigma), 7-deaza-dGTP (Roche) | Disrupt secondary structures, equalize base stability, and reduce template melting temperature [4] |
| Optimization Tools | Gradient Thermal Cycler, MgClâ‚‚ titration series, NEB Tm Calculator | Enable systematic optimization of critical parameters without requiring multiple separate experiments [1] [2] |
| Enhanced Buffer Systems | GC Buffer (NEB), Q5 High GC Enhancer (NEB), Commercial master mixes | Pre-formulated combinations of optimal salts, additives, and stabilizers specifically designed for challenging amplifications [1] |
Advanced Combination Strategy Diagram
Additive Implementation Strategy
The combination of DMSO and betaine represents a powerful approach in GC-rich PCR optimization research. Their synergistic action addresses both the structural challenges (through DMSO) and thermodynamic barriers (through betaine) that impede conventional amplification [4]. This combination strategy has proven essential for amplifying clinically relevant targets including promoter regions of housekeeping genes, tumor suppressor genes, and specific disease markers with GC content exceeding 80% [1] [4] [5]. When implementing this approach, researchers should systematically optimize concentrations while considering template-specific characteristics, as there is no universal solution that works identically for all GC-rich amplicons [1] [2].
Dimethyl sulfoxide (DMSO) stands as one of the most versatile solvents in biological research, serving dual roles as both a powerful cryoprotectant and an effective penetration enhancer. Beyond these established applications, DMSO functions as a potent disruptor of molecular secondary structures, a property that makes it invaluable in molecular biology techniques, particularly when dealing with challenging DNA templates. Its small amphiphilic nature allows it to interact with both polar and nonpolar compounds, making it miscible in a wide range of organic solvents as well as water [8]. When combined with natural osmolyte betaine in PCR applications, DMSO exhibits remarkable efficacy in amplifying GC-rich sequences that are otherwise refractory to conventional amplification methods. This technical guide explores the molecular mechanisms behind DMSO's structure-disrupting properties and provides practical protocols for researchers leveraging DMSO-betaine combinations to overcome experimental challenges in amplification of complex DNA sequences.
Molecular Mechanisms: How DMSO Disrupts Secondary Structures
Interaction with Lipid Membranes
DMSO exhibits three distinct concentration-dependent modes of action when interacting with phospholipid membranes, as revealed through atomic-scale molecular dynamics simulations [9]. At low concentrations (typically < 10%), DMSO induces membrane thinning and increases fluidity of the membrane's hydrophobic core by intercalating between lipid molecules. At moderate concentrations (approximately 10-20%), DMSO facilitates the formation of transient water pores through the membrane, explaining its significant enhancement of membrane permeability to hydrophilic molecules. At high concentrations (> 20%), DMSO causes desorption of individual lipid molecules from the membrane, ultimately leading to complete disintegration of the bilayer structure. These membrane-disrupting properties directly facilitate DMSO's role as a penetration enhancer and cryoprotectant in cellular applications.
Effects on Proteins and Nucleic Acids
The structure-disrupting capability of DMSO extends beyond lipid membranes to proteins and nucleic acids. Spectroscopy studies reveal that DMSO progressively disrupts the native tertiary structure of hemoglobin, with complete disruption occurring at approximately 50% DMSO concentration [10]. The mechanism involves breaking hydrogen bonds between prosthetic groups and nearby surface amino acid residues, while simultaneously disorganizing the hydrophobic interior of the protein. When DMSO concentration increases to 57%, the native α-helical secondary structure is lost, leading to aggregation and formation of intermolecular β-sheets [10]. For nucleic acids, DMSO effectively disrupts secondary structure formation in GC-rich DNA by interfering with the strong hydrogen bonding between guanine and cytosine bases, particularly those involving the N-7 position of guanine rings that contribute to complex intra- and interstrand folding [11].
Table 1: Concentration-Dependent Effects of DMSO on Biological Structures
| DMSO Concentration | Effect on Lipid Membranes | Effect on Proteins | Effect on Nucleic Acids |
|---|---|---|---|
| Low (< 10%) | Membrane thinning & increased fluidity | Partial tertiary structure disruption | Mild secondary structure destabilization |
| Moderate (10-20%) | Transient water pore formation | Significant tertiary structure loss | Effective secondary structure disruption |
| High (> 20%) | Lipid desorption & bilayer disintegration | Secondary structure loss & aggregation | Denaturation and structural unfolding |
Experimental Protocols: DMSO-Betaine Combinations for GC-Rich PCR
Standard PCR Protocol with DMSO and Betaine
The powerful combination of DMSO and betaine has proven essential for amplifying GC-rich DNA sequences with GC content ranging from 67% to 79% [4]. The following protocol is adapted from established methodologies that successfully amplified challenging regions of the RET, LMX1B, and PHOX2B genes:
Reaction Setup: Prepare a 25 μL PCR reaction containing:
- 1X PCR buffer (commercial formulation)
- 2.0-2.5 mM MgClâ‚‚ (concentration may require optimization)
- 200 μM of each dNTP (dATP, dCTP, dTTP, dGTP)
- 10-20 pmol of each forward and reverse primer
- 1.25 units of DNA polymerase (standard Taq or Gold Taq)
- 100 ng of genomic DNA template
Additive Incorporation:
- 1.3 M betaine (final concentration)
- 5% DMSO (v/v, final concentration)
- Optional: 50 μM 7-deaza-dGTP may be added for extremely challenging templates [4]
Thermal Cycling Conditions:
- Initial denaturation: 94°C for 3-5 minutes
- 25-40 cycles of:
- Denaturation: 94°C for 10-30 seconds
- Annealing: 55-60°C for 30 seconds (temperature primer-dependent)
- Extension: 68-72°C for 45-60 seconds per kb
- Final extension: 68-72°C for 5-10 minutes
Product Analysis: Analyze 5 μL of PCR product by agarose gel electrophoresis (1.2-2.0% depending on product size) [4].
Troubleshooting Common Issues
Table 2: Troubleshooting DMSO-Betaine PCR Amplification
| Problem | Potential Cause | Solution |
|---|---|---|
| No amplification | Excessive secondary structure | Increase DMSO to 7-10% or combine with 7-deaza-dGTP |
| Nonspecific products | Betaine concentration suboptimal | Titrate betaine (0.5-2.0 M) or increase annealing temperature |
| Preferential amplification of shorter alleles | Differential secondary structure | Use all three additives: DMSO, betaine, and 7-deaza-dGTP [4] |
| Reduced polymerase activity | DMSO inhibition | Use a specialized polymerase tolerant to organic solvents |
| Smear of products | Over-cycling | Reduce cycle number or decrease magnesium concentration |
Research Reagent Solutions
Table 3: Essential Reagents for DMSO-Mediated Structure Disruption
| Reagent | Function | Working Concentration | Mechanism of Action |
|---|---|---|---|
| DMSO | Secondary structure disruptor | 1-10% (typically 5%) | Disrupts hydrogen bonding and base stacking interactions |
| Betaine | Isostabilizing agent | 0.5-2.0 M (typically 1.3 M) | Equilibrates Tm differences between AT and GC base pairs |
| 7-deaza-dGTP | Guanine analog | 50 μM (with 150 μM dGTP) | Reduces hydrogen bonding capacity of guanine residues |
| High-Fidelity Polymerase | Enzyme for amplification | 0.5-2.5 units/50 μL reaction | Maintains activity in presence of additives |
| Magnesium Chloride | Cofactor | 1.5-4.0 mM | Optimizes polymerase activity; may require adjustment with additives |
Experimental Workflow and Mechanism Visualization
The following diagram illustrates the strategic workflow for applying DMSO and betaine to overcome secondary structure challenges in PCR amplification:
Frequently Asked Questions
Q1: What is the optimal concentration of DMSO for PCR applications? For most PCR applications involving GC-rich templates, 5% DMSO (v/v) provides an effective balance between secondary structure disruption and polymerase compatibility. However, concentration optimization between 1-10% is recommended for specific applications, as excessive DMSO can inhibit polymerase activity [6] [4].
Q2: Why are DMSO and betaine often used together? DMSO and betaine operate through complementary mechanisms. DMSO directly disrupts hydrogen bonding in secondary structures, while betaine acts as an isostabilizing agent that equalizes the melting temperature differences between AT and GC base pairs [11]. This combination addresses both the structural and thermodynamic challenges of GC-rich sequences.
Q3: Can DMSO affect biological systems beyond nucleic acid secondary structure? Yes, DMSO has broad effects on biological systems. At concentrations above 1%, DMSO induces morphological and physiological alterations in zebrafish embryos, including curved tail, heart edema, and changes in heart beating frequency [8]. In proteins, DMSO disrupts tertiary structure even at relatively low concentrations, with complete disruption of hemoglobin's native structure occurring at 50% DMSO [10].
Q4: How does DMSO compare to other PCR enhancers? DMSO remains one of the most effective and widely used additives for GC-rich templates, particularly when combined with betaine. While other additives like formamide, glycerol, and polyethylene glycol can also improve amplification, the DMSO-betaine combination has proven uniquely effective for sequences with GC content exceeding 70% [11] [4].
Q5: Are there any special considerations when using DMSO in experimental controls? Yes, it is critical to maintain equivalent DMSO concentrations across all experimental and control reactions, as DMSO concentration directly influences membrane permeability, protein structure, and nucleic acid stability [9] [8] [10]. Vehicle controls should match the DMSO concentration used in treatment conditions.
Betaine is a powerful isostabilizing agent that eliminates the base pair composition dependence of DNA melting. At a concentration of approximately 5.2 M, betaine makes AT and GC base pairs equally stable without significantly altering the B-form conformation of double-stranded DNA or greatly changing DNA's behavior as a polyelectrolyte [12]. This property is crucial for PCR amplification of GC-rich templates, where stable secondary structures often form and hinder polymerase progression. By equalizing the stability of AT and GC base pairs, betaine reduces the formation of these secondary structures, facilitating more efficient and specific DNA amplification [11] [13].
FAQs: Understanding Betaine and DMSO in PCR
What is the fundamental mechanism by which betaine functions as a DNA isostabilizer?
Betaine, an amino acid analog, exists as a zwitterion near neutral pH. It exerts its isostabilizing effect by equilibrating the differential melting temperature (Tm) between AT and GC base pairings [11] [13]. In doing so, it effectively eliminates the base pair composition dependence of DNA melting, meaning that at its isostabilizing concentration (approximately 5.2 M), AT and GC base pairs become equally stable [12]. This promotes strand separation and disrupts the secondary structures that are common in GC-rich regions and that typically block polymerase activity during PCR.
How does DMSO complement the action of betaine in PCR amplification?
While betaine acts as an isostabilizer, DMSO (Dimethyl sulfoxide) functions by disrupting inter- and intrastrand re-annealing of DNA [11] [13]. Recent single-molecule studies have shown that DMSO linearly decreases the bending persistence length of DNA (by approximately 0.43% per %-DMSO up to 20%) and causes a moderate compaction of DNA conformations [14]. This means DMSO makes DNA more flexible and less likely to form rigid secondary structures. When used together, betaine and DMSO attack the problem of GC-rich amplification from two different angles: betaine normalizes the melting temperature across the DNA molecule, while DMSO directly destabilizes secondary structures.
When should I use betaine and DMSO separately, and when should I combine them?
Evidence suggests a sequential optimization strategy is most effective:
- Use DMSO by default: One extensive study on plant ITS2 DNA barcodes found that 5% DMSO alone achieved a 91.6% PCR success rate [15].
- Substitute with betaine if DMSO fails: In the same study, the one sample that did not amplify with DMSO was successfully amplified by adding 1 M betaine instead [15].
- Combine with caution: Combining DMSO and betaine in the same reaction did not improve the PCR success rate in the ITS2 study and is therefore not recommended as a first-line approach [15]. However, for extremely challenging templates, a powerful combination includes 1.3 M betaine, 5% DMSO, and 50 μM 7-deaza-dGTP, which was essential for amplifying sequences with GC content from 67% to 79% [4].
What are the optimal concentrations for these additives, and can they inhibit PCR?
Using these additives at incorrect concentrations can inhibit the PCR reaction. The table below summarizes the typical working concentrations and critical inhibition thresholds.
Table 1: Recommended Concentrations and Inhibition Thresholds for PCR Additives
| Additive | Common Working Concentration | Reported Inhibitory Concentration | Key Considerations |
|---|---|---|---|
| Betaine | 0.5 M - 2.5 M [6] | >2.5 M (context-dependent) | Isostabilizing concentration is ~5.2 M, but PCR typically uses lower concentrations [12]. |
| DMSO | 1% - 10% [6] | >10% [16] | Linearly reduces DNA persistence length; >20% induces more significant structural changes [14]. |
| 7-deaza-dGTP | 50 μM [4] | N/A | Often used in combination with betaine and DMSO for the most challenging templates [4]. |
Troubleshooting Guides
Problem: Failure to Amplify a GC-Rich DNA Target
Potential Causes and Solutions:
Ineffective disruption of secondary structures:
Additive inhibition:
- Solution: Ensure DMSO concentration does not exceed 10%. Titrate betaine and DMSO concentrations to find the optimal balance for your specific template [16].
Suboptimal cycling conditions:
- Solution: Use a higher annealing temperature and a longer extension time. A "touchdown" PCR protocol, starting with a higher annealing temperature and gradually reducing it, can also improve specificity [16].
Problem: Non-Specific Amplification or Smearing
Potential Causes and Solutions:
Excessive additive or reagent concentration:
- Solution: Reduce the concentration of DMSO, betaine, Mg2+, dNTPs, or polymerase. High concentrations of these reagents can reduce fidelity and cause smearing [16].
Annealing temperature too low:
- Solution: Optimize the annealing temperature by increasing it in 2-5°C increments. Using a hot-start polymerase can also prevent mis-priming at lower temperatures [16].
Too many PCR cycles:
- Solution: Reduce the number of amplification cycles, typically to between 20-40 [16].
Experimental Protocols
Protocol 1: Basic PCR with Additives for GC-Rich Templates
This protocol is adapted from a standard basic PCR guide and incorporates additive options [6].
Research Reagent Solutions:
- Template DNA: 1-1000 ng (10^4 to 10^7 molecules) of genomic DNA.
- 10X PCR Buffer: Supplied with the DNA polymerase.
- MgClâ‚‚ Solution: 25 mM stock (if not present in the buffer). Final concentration typically 1.5-4.0 mM.
- dNTP Mix: 10 mM total (2.5 mM of each dATP, dCTP, dGTP, dTTP).
- Primers: 20 μM stock each.
- Taq DNA Polymerase: 0.5-2.5 units per 50 μL reaction.
- Sterile Distilled Water: Q.S. to final volume.
- Additive Stocks: 100% DMSO, 5M Betaine, 10 mM 7-deaza-dGTP.
Table 2: Sample 50 μL PCR Reaction Setup with Additives
| Reagent | Final Concentration | Volume for 50 μL Reaction |
|---|---|---|
| 10X PCR Buffer | 1X | 5 μL |
| dNTP Mix (10 mM) | 200 μM | 1 μL |
| MgCl₂ (25 mM) | 1.5 - 4.0 mM | Variable (e.g., 3.2 μL for 1.6 mM) |
| Forward Primer (20 μM) | 0.4 μM | 1 μL |
| Reverse Primer (20 μM) | 0.4 μM | 1 μL |
| Template DNA | Variable | Variable (e.g., 0.5 μL of 2 ng/μL) |
| Additive: DMSO (100%) | 5% | 2.5 μL |
| - OR - Additive: Betaine (5M) | 1 M | 10 μL |
| Taq Polymerase | 0.5 - 2.5 U | 0.5 - 1 μL |
| Sterile Water | Q.S. to 50 μL | ~ 25.3 μL (adjust based on additive) |
Procedure:
- Prepare Master Mix: Thaw all reagents on ice. Combine all components except the template DNA in a sterile 1.8 mL microcentrifuge tube. Mix gently by pipetting up and down.
- Aliquot: Dispense the master mix into individual 0.2 mL thin-walled PCR tubes.
- Add Template: Add template DNA to each experimental tube. For a negative control, add an equivalent volume of sterile water.
- Thermal Cycling: Place tubes in a thermal cycler and run with the following typical program:
- Initial Denaturation: 94°C for 3-5 minutes.
- Amplification (25-40 cycles):
- Denature: 94°C for 15-45 seconds.
- Anneal: 55-65°C for 30 seconds (optimize for your primers).
- Extend: 68-72°C for 1 minute per kb.
- Final Extension: 68-72°C for 5-10 minutes.
- Hold: 4°C ∞.
Protocol 2: Advanced Three-Additive Mixture for Highly Refractory Templates
This protocol is adapted from research that successfully amplified DNA sequences with 67-79% GC content [4].
Research Reagent Solutions:
- All standard PCR reagents (as in Protocol 1).
- Betaine Stock: 5M solution.
- DMSO: 100%.
- 7-deaza-dGTP Stock: 10 mM solution.
Procedure:
- Prepare Reaction Mix: Set up a 25 μL PCR reaction containing:
- 1X PCR Buffer (supplemented with 2.5 mM MgClâ‚‚).
- 200 μM dATP, dCTP, dTTP.
- 50 μM 7-deaza-dGTP (substitutes for a portion of dGTP).
- 10 nmol of each primer.
- 100 ng of genomic DNA.
- 1.3 M Betaine (e.g., 6.5 μL of a 5M stock).
- 5% DMSO (e.g., 1.25 μL of 100% DMSO).
- 1.25 units of Taq DNA polymerase.
- Thermal Cycling: Use a "slow-start" or "hot-start" protocol with a high annealing temperature. An example profile:
- Initial Denaturation: 94°C for 3-5 minutes.
- Amplification (25-35 cycles):
- Denature: 94°C for 10-30 seconds.
- Anneal/Extend: 68°C for 3 minutes (a combined step can enhance efficiency for short products).
- Final Extension: 72°C for 5-10 minutes.
- Hold: 4°C ∞.
Workflow and Decision Diagram
The diagram below outlines a systematic workflow for troubleshooting PCR amplification of GC-rich sequences using betaine, DMSO, and other additives.
Key Research Reagent Solutions
The following table details the essential reagents used in the experiments and protocols cited in this guide.
Table 3: Essential Research Reagents for PCR of GC-Rich DNA
| Reagent | Function / Mechanism of Action | Key Experimental Use Cases |
|---|---|---|
| Betaine | Isostabilizing agent; equalizes Tm of AT and GC base pairs, disrupting secondary structures [11] [12]. | Used at 1 M to amplify plant ITS2 barcodes after DMSO failed [15]. Used at 1.3 M in a triple-additive mix for GC-rich human genes [4]. |
| DMSO (Dimethyl Sulfoxide) | Disrupts inter- and intrastrand DNA re-annealing; reduces DNA persistence length and melting temperature [11] [14]. | Used at 5% as a default additive, achieving 91.6% success rate for plant ITS2 barcodes [15]. |
| 7-deaza-dGTP | dGTP analog that incorporates into DNA and reduces hydrogen bonding, thereby weakening secondary structure formation [4]. | Critical component (at 50 µM) of a triple-additive mix for amplifying human genes with 67-79% GC content [4]. |
| High-Fidelity DNA Polymerase | Thermostable enzyme with proofreading activity for accurate amplification of complex templates. | Used in de novo synthesis of GC-rich constructs (IGF2R, BRAF) with DMSO/betaine [11]. |
| dNTPs | Nucleotide building blocks (dATP, dCTP, dGTP, dTTP) for DNA synthesis. | Standard component of all PCR reactions; concentration typically 200 µM each [6]. |
While both DMSO (Dimethyl Sulfoxide) and betaine are powerful additives for amplifying difficult, GC-rich DNA templates, combining them in a single PCR reaction is not universally recommended. Their synergistic effect is highly specific to particular template sequences and conditions. Indiscriminate combination can lead to failed amplification, as their mechanisms can interfere with each other or with the polymerase, especially at non-optimized concentrations. This guide provides troubleshooting and protocols for determining the correct application of these reagents.
FAQ: Solving Common DMSO and Betaine Problems
Why did my PCR fail when I combined DMSO and betaine?
The combination of DMSO and betaine is not a universal solution and can fail for several reasons:
- Mechanistic Interference: DMSO and betaine work through different mechanisms to reduce DNA secondary structures. Betaine acts as a isostabilizer, equilibrating the melting temperatures of GC and AT base pairs [13] [11]. DMSO disrupts inter- and intrastrand re-annealing [13] [11]. In some cases, these distinct actions can interfere with one another or adversely affect polymerase activity [17].
- Inhibitory Concentrations: The beneficial effects of these additives are concentration-dependent. High concentrations, especially in combination, can inhibit Taq polymerase. One study found that while 7% DMSO worked well, a combination of 10% DMSO and 15% glycerol blocked amplification entirely [18].
- Template Specificity: Success with the combination is highly template-specific. For example, one study demonstrated that a triple mixture of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP was essential for amplifying specific GC-rich disease genes (GC content of 67-79%) [4]. However, for other templates, like the ultra-stable inverted terminal repeats (ITRs) of adeno-associated virus (AAV), neither DMSO nor betaine—alone or combined—showed any improving effect [17].
When should I consider combining DMSO and betaine?
Consider testing the combination only after single additives have failed, and only if you are working with an exceptionally challenging GC-rich template (e.g., >70% GC content) that is known to form stable secondary structures [4]. The combination has been critical for sequencing specific promoter regions and exons in genes like RET, LMX1B, and PHOX2B [4]. Always include a systematic optimization experiment with controls.
What is a safer alternative to combining them?
A more reliable strategy is to test DMSO and betaine separately first. Begin by titrating each additive individually to find the optimal concentration for your specific template before attempting to combine them. For the most challenging structures, investigate novel methods like "disruptor" oligonucleotides, which are specifically designed to bind and unwind stable intramolecular structures and have succeeded where traditional additives failed [17].
Experimental Protocols & Data
Protocol 1: Testing Additives Individually and in Combination
This protocol is adapted from methods used to amplify the GC-rich RET promoter region [4].
- Reaction Setup: Prepare a master mix for a standard 25 µL PCR reaction containing your template DNA, primers, dNTPs, and a standard buffer.
- Additive Titration: Aliquot the master mix into separate tubes and supplement them as follows:
- Control: No additives.
- DMSO only: Test a range from 5% to 10% (v/v) [18].
- Betaine only: Test a range from 1 M to 2 M [18].
- Combination: Test combinations of the most effective individual concentrations (e.g., 5% DMSO with 1.5 M betaine).
- Reference Combination: Include the "powerful mixture" of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP as a positive control for challenging templates [4].
- Thermal Cycling: Run the PCR using your standard cycling conditions, though you may need to adjust the annealing temperature based on the additives used.
- Analysis: Analyze the results by agarose gel electrophoresis to assess specificity and yield.
Protocol 2: The "Powerful Mixture" for Intractable GC-Rich Templates
For templates that remain unamplifiable, use this established protocol [4].
- Final Concentrations in PCR Mix:
- Betaine: 1.3 M
- DMSO: 5% (v/v)
- 7-deaza-dGTP: 50 µM (Note: This is a partial substitution for dGTP. Use a dNTP mix where 50 µM of the dGTP is replaced with 7-deaza-dGTP.)
- Primers: 10 nmol each
- Taq Polymerase: 1.25 units
- MgClâ‚‚: 2.5 mM
The table below summarizes key findings from research on PCR additives, demonstrating that outcomes depend on the specific template and conditions.
| Template (GC Content) | Effective Additive(s) | Ineffective/Blocking Additive(s) | Key Findings | Source |
|---|---|---|---|---|
| RET promoter (79% GC) | 1.3 M Betaine + 5% DMSO + 50 µM 7-deaza-dGTP | Betaine + DMSO (without 7-deaza-dGTP) | The specific triple combination was essential for a unique, specific product. | [4] |
| rAAV ITR (Ultra-stable) | Disruptor oligonucleotides | DMSO and/or Betaine | DMSO and betaine showed no improving effect on these ultra-stable structures. | [17] |
| EGFR promoter (GC-rich) | 7% DMSO; 10% Glycerol; 1-2 M Betaine | 10% DMSO + 15% Glycerol | Single additives worked, but one specific combination blocked amplification. | [18] |
| IGF2R & BRAF (GC-rich) | DMSO or Betaine (during amplification) | DMSO or Betaine (during assembly) | Additives greatly improved amplification, but provided no benefit during gene assembly steps. | [13] [11] |
The Scientist's Toolkit: Research Reagent Solutions
| Reagent | Function in PCR | Key Consideration |
|---|---|---|
| DMSO (Dimethyl Sulfoxide) | Disrupts secondary structures by preventing inter- and intrastrand re-annealing of DNA [13] [11]. | Effective concentration range is typically 5-10%. Higher concentrations can inhibit polymerase [18]. |
| Betaine (Monohydrate) | Equalizes the melting temperature (Tm) of GC and AT base pairs, facilitating the denaturation of GC-rich regions [13] [11]. | Often used in a concentration range of 1-2 M. Can be used alone or in specific combinations [4] [18]. |
| 7-deaza-dGTP | A dGTP analog that reduces hydrogen bonding, thereby weakening GC interactions and destabilizing secondary structures [4]. | Often used as a partial substitute for dGTP (e.g., 50 µM). Critical for some of the most challenging templates [4]. |
| Disruptor Oligos | Novel oligonucleotides designed to bind and physically unwind stable intramolecular secondary structures in the template [17]. | A sequence-specific solution that can work where chemical additives fail, such as on AAV ITRs [17]. |
| N-(4-cyanophenyl)-2-methylprop-2-enamide | N-(4-cyanophenyl)-2-methylprop-2-enamide | N-(4-cyanophenyl)-2-methylprop-2-enamide for research. Molecular Formula C11H12N2O, MW 188.23. For Research Use Only. Not for human or veterinary use. |
| N-(2,2-dimethoxyethyl)cyclohexanamine | N-(2,2-dimethoxyethyl)cyclohexanamine, CAS:99863-45-3, MF:C10H21NO2, MW:187.28 g/mol | Chemical Reagent |
Workflow: Choosing the Right PCR Additive Strategy
The following diagram illustrates the decision-making process for troubleshooting a PCR with a suspected secondary structure problem.
Summary of the additive selection workflow.
Key Technical Notes
- Contamination Control: Always use a unidirectional workflow, physically separating pre- and post-PCR areas to prevent amplicon contamination, which is a major cause of false positives [19] [20].
- Reagent Quality: Store oligonucleotides and reagents in single-use aliquots to minimize freeze-thaw cycles and contamination risk [20].
- PCR Tubes: Use high-quality, thin-walled PCR tubes for optimal thermal conductivity. Reusing tubes is generally not recommended due to high risks of contamination and physical degradation [21] [22].
Polymersse Chain Reaction (PCR) is a foundational technique in molecular biology, yet the amplification of DNA with high GC-content (>60%) presents a significant challenge for researchers in fields ranging from diagnostics to synthetic biology. The strong hydrogen bonding and formation of stable secondary structures in GC-rich sequences hinder polymerase progression and primer annealing, leading to amplification failure, nonspecific products, or low yield. Within this context, the combination of dimethyl sulfoxide (DMSO) and betaine has emerged as a powerful and cost-effective strategy to overcome these obstacles. This technical support center provides troubleshooting guides and detailed protocols to help researchers reliably amplify difficult templates, thereby supporting advanced applications in DNA barcoding and de novo gene synthesis.
FAQs: Combining DMSO and Betaine in PCR
1. Why should I combine DMSO and betaine instead of using just one?
While both additives facilitate the amplification of GC-rich DNA, they operate through distinct yet complementary mechanisms. Using them together can produce a synergistic effect that is often essential for successfully amplifying the most challenging templates [4].
- Betaine acts as an isostabilizing agent. It equilibrates the differential melting temperature (Tm) between AT and GC base pairs by interacting with the negatively charged groups on the DNA strand, which reduces electrostatic repulsion and prevents the formation of secondary structures [23] [24].
- DMSO primarily functions by disrupting hydrogen bonding and intrastrand base pairing. It interacts with water molecules around the DNA, reducing the DNA's Tm and helping to keep the DNA in a single-stranded state, thus preventing the reformation of secondary structures during the reaction [13] [23].
Single additives can reduce nonspecific background, but may be insufficient for specific amplification. Research has demonstrated that for several disease genes with GC content ranging from 67% to 79%, a combination of all three additives—betaine, DMSO, and 7-deaza-dGTP—was essential to achieve a unique, specific PCR product [4].
2. What are the recommended starting concentrations for this combination?
A typical starting point for a combined additive PCR is provided below. These concentrations should be optimized for your specific template and primer set.
| Additive | Final Concentration | Role in PCR |
|---|---|---|
| Betaine | 1.0 M - 1.3 M [4] [24] | Reduces DNA secondary structure formation, enhances specificity [23]. |
| DMSO | 5% - 10% (v/v) [4] [18] [24] | Disrupts hydrogen bonding, lowers DNA melting temperature [23]. |
| 7-deaza-dGTP | 50 μmol/L (as a partial or full substitute for dGTP) [4] | Reduces hydrogen bonding in GC-rich regions by base modification. |
Note: Higher concentrations of DMSO (e.g., >10%) can inhibit Taq polymerase, so a balance must be struck [16]. Betaine is typically used at high molar concentrations but is well-tolerated by most polymerases.
3. How do other common additives compare to the DMSO/Betaine combination?
A systematic 2024 study compared the effectiveness of nine PCR enhancers on DNA fragments with varying GC content. The results below show the Cycle Threshold (Ct) values, where a lower Ct indicates more efficient amplification [24].
Table: Comparison of PCR Enhancer Efficiency on Different GC-Content Templates (Ct Values)
| Enhancer | Concentration | Moderate GC (53.8%) | High GC (68.0%) | Super High GC (78.4%) |
|---|---|---|---|---|
| Control | - | 15.84 | 15.48 | 32.17 |
| DMSO | 5% | 16.68 | 15.72 | 17.90 |
| Betaine | 1.0 M | 16.35 | 15.09 | 16.71 |
| Formamide | 5% | 18.08 | 15.44 | 16.32 |
| Glycerol | 10% | 16.49 | 15.44 | 17.18 |
| Sucrose | 0.4 M | 16.39 | 15.03 | 16.67 |
| Trehalose | 0.4 M | 16.43 | 15.15 | 16.91 |
The data demonstrates that while most enhancers can improve the amplification of high-GC targets, betaine consistently delivers some of the lowest Ct values (highest efficiency) for the most challenging "super high" GC-rich template [24]. Combinations like betaine with sucrose also show promising results with minimal negative effects on normal PCR [24].
4. Do I need to adjust my PCR cycling parameters when using DMSO and betaine?
Yes. The presence of DMSO and betaine lowers the melting temperature (Tm) of the DNA template and the primer-template complex [25]. Consequently, you should adjust your protocol as follows:
- Annealing Temperature: Lower the annealing temperature by 5–6°C, especially if using DMSO at a concentration of around 10% [25].
- Denaturation: You may need to increase the denaturation time and/or temperature for GC-rich templates, though the additives themselves enhance strand separation, which can sometimes make prolonged denaturation unnecessary [25] [26].
- Extension: Standard extension times (e.g., 1 min/kb for Taq polymerase) typically apply, but ensure a final extension step of 5–15 minutes to complete all products [25].
Troubleshooting Guide
| Problem | Possible Cause | Recommended Solution |
|---|---|---|
| No Amplification | Additives inhibit polymerase; over-optimized conditions. | Titrate additive concentrations downward. Use a polymerase known for high processivity and tolerance to co-solvents. Ensure fresh, high-quality DNA template [26] [27]. |
| Non-specific Bands/Smearing | Annealing temperature too low; insufficient additive concentration. | Increase the annealing temperature in 2–3°C increments. Optimize Mg2+ concentration. Use a hot-start polymerase to prevent primer-dimer formation and non-specific priming at lower temperatures [16] [26]. |
| Low Yield | Suboptimal concentration of DMSO/betaine; poor primer design; insufficient enzyme. | Systematically test a range of DMSO (2%-10%) and betaine (0.5 M-2 M) concentrations. Verify primer specificity and Tm. Increase the number of PCR cycles if the template is scarce [18] [26]. |
| Inconsistent Results | Non-homogeneous reagent mixing; pipetting errors with viscous additives. | Thoroughly mix the reagent stocks and the prepared PCR reaction. Use master mixes to minimize pipetting variability. Aliquot betaine and DMSO stocks to ensure consistency [26]. |
Experimental Protocol: Amplifying GC-Rich Targets with DMSO and Betaine
The following protocol is adapted from published studies that successfully amplified GC-rich sequences (67-79% GC) from human disease-related genes [4] [28].
1. Reagent Setup
Prepare a PCR master mix on ice with the following components for a 25 µL reaction:
| Component | Final Concentration/Amount |
|---|---|
| PCR Buffer (with MgClâ‚‚) | 1X |
| MgClâ‚‚ (if not in buffer) | 1.5 - 2.5 mM (optimize) |
| dNTP Mix | 200 µM each |
| Forward Primer | 0.2 - 1.0 µM |
| Reverse Primer | 0.2 - 1.0 µM |
| Template DNA | 50 - 200 ng (genomic DNA) |
| Betaine | 1.0 - 1.3 M |
| DMSO | 5% (v/v) |
| Taq DNA Polymerase | 1.0 - 1.25 units |
| Water, Nuclease-free | to 25 µL |
Note: For extremely problematic templates, consider a partial substitution of dGTP with 7-deaza-dGTP (e.g., 50 µM) [4].
2. Thermal Cycling Conditions
Use the following cycling parameters as a starting point in a thermal cycler:
- Initial Denaturation: 94–95°C for 3–5 minutes
- Amplification (25–35 cycles):
- Denaturation: 94°C for 30 seconds
- Annealing: 55–68°C for 30 seconds (Optimize temperature based on primers and additives)
- Extension: 72°C for 1 minute per kb of amplicon
- Final Extension: 72°C for 5–10 minutes
- Hold: 4°C
Research Reagent Solutions
Table: Essential Materials for PCR of GC-Rich Templates
| Item | Function | Example/Note |
|---|---|---|
| Betaine (Monohydrate) | Isostabilizing agent that reduces DNA secondary structure. | Use betaine monohydrate instead of hydrochloride to avoid pH changes [23]. |
| DMSO (Molecular Biology Grade) | Disrupts hydrogen bonding, lowers DNA Tm. | Higher grades prevent chemical contaminants from inhibiting the reaction. |
| 7-deaza-dGTP | dGTP analog that reduces hydrogen bonding in GC-rich regions. | Used as a partial or full substitute for dGTP in challenging cases [4]. |
| Hot-Start DNA Polymerase | Reduces non-specific amplification and primer-dimer formation at low temperatures. | Essential for maintaining reaction specificity when using lower annealing temperatures [26]. |
| GC Enhancer Solution | Commercial formulations designed to improve amplification of GC-rich targets. | e.g., Platinum GC Enhancer (Thermo Fisher) [16]. |
Workflow and Mechanism Diagrams
The following diagram illustrates the experimental workflow for optimizing PCR with DMSO and betaine.
Optimization Workflow for GC-Rich PCR
The following diagram illustrates the mechanistic action of DMSO and betaine on DNA secondary structures.
Mechanism of DMSO and Betaine
Practical Protocols: Step-by-Step Guide to Using DMSO and Betaine in Your Reactions
Polymerase Chain Reaction (PCR) is a foundational technique in molecular biology, yet amplifying GC-rich DNA sequences (typically defined as those with a guanine-cytosine content of 60% or greater) presents a significant challenge [28] [29]. The strong hydrogen bonding between G and C bases (three bonds versus two for A-T pairs) increases the thermostability of the DNA duplex, often leading to incomplete denaturation and the formation of stable secondary structures, such as hairpins [29]. These structures can hinder primer annealing and cause DNA polymerases to stall, resulting in poor amplification efficiency, low yield, or complete PCR failure [28] [13].
To overcome these obstacles, scientists routinely employ PCR additives. Among the most effective are dimethyl sulfoxide (DMSO) and betaine [13]. These chemicals work through different but complementary mechanisms to destabilize DNA secondary structures and facilitate the amplification of difficult templates. This guide provides a detailed technical overview of how to combine DMSO and betaine in PCR research, offering optimized protocols and troubleshooting advice for researchers and drug development professionals.
FAQs on DMSO and Betaine Use in PCR
1. How do DMSO and betaine improve PCR amplification of GC-rich sequences?
DMSO and betaine are known as isostabilizing agents because they help equalize the stability of DNA duplexes with varying base compositions.
- Mechanism of DMSO: DMSO interacts with water molecules and the DNA strand, reducing the stability of hydrogen bonds. This action lowers the melting temperature (Tm) of DNA, making it easier to denature stable secondary structures that would otherwise block the polymerase [30]. However, DMSO can also reduce Taq polymerase activity, making concentration optimization critical [30].
- Mechanism of Betaine: Betaine (an amino acid analog) reduces the formation of DNA secondary structures by diminishing the electrostatic repulsion between DNA strands [30]. It is particularly effective because it eliminates the dependence of DNA melting on base pair composition, effectively equalizing the contribution of GC and AT base pairs to the overall duplex stability [13] [30]. This makes it especially useful for amplifying GC-rich sequences.
2. Why are 5% DMSO and 1M Betaine recommended as starting points?
The combination of 5% DMSO and 1M betaine is a widely supported and effective starting concentration for troubleshooting GC-rich PCRs. Research has demonstrated that this combination, or similar ratios, significantly improves target product specificity and yield during the amplification of challenging constructs [31] [13]. One study specifically found that a combination of 10% DMSO with 15% glycerol was effective, while other combinations failed, underscoring the importance of systematic optimization [31]. Starting with 5% DMSO and 1M betaine provides a balanced approach that harnesses the benefits of both additives without immediately introducing potential inhibition from higher concentrations.
3. Can DMSO and betaine be used together?
Yes, DMSO and betaine are highly compatible and can be used together in a single PCR reaction [13]. Their mechanisms of action are complementary, and their combined use has been reported to allow for the production of a wide variety of GC-rich gene constructs without the need for extensive protocol modifications [13].
4. What are the potential pitfalls of using these additives?
The primary pitfall is using excessively high concentrations, which can inhibit the PCR reaction.
- DMSO: At high concentrations (typically >10%), DMSO can significantly inhibit Taq DNA polymerase activity [16] [30].
- Betaine: While generally well-tolerated, the specific concentration must be controlled to avoid negative effects on the PCR [30]. Furthermore, it is recommended to use betaine or betaine monohydrate rather than betaine hydrochloride, as the latter may affect the pH of the reaction and thus enzyme activity [30].
Troubleshooting Guide
The following table outlines common problems encountered when amplifying GC-rich templates and how DMSO, betaine, and other parameters can be adjusted to resolve them.
| Problem Observed | Potential Causes | Recommended Solutions & Adjustments |
|---|---|---|
| No Product or Weak Yield | • Polymerase stalling on secondary structures• Additive concentration too high (inhibition)• Annealing temperature too high | • Additives: Test 1-1.7M betaine and/or 2-10% DMSO [32] [30]. Start with 5% DMSO + 1M betaine [31] [13].• Polymerase: Use a polymerase specifically optimized for GC-rich templates [29].• Thermal Cycling: Increase denaturation temperature or use a longer denaturation time [16]. |
| Non-specific Bands or Smearing | • Non-specific primer binding• Additive concentration too low• Mg2+ concentration too high | • Additives: Introduce DMSO or formamide to increase primer stringency [29] [30].• Thermal Cycling: Increase the annealing temperature in 2-5°C increments [16] [29].• Mg2+: Optimize Mg2+ concentration, trying lower values (e.g., 1.0-4.0 mM) [16] [29]. |
| High Molecular Weight Smear | • Too many PCR cycles• Excessive template DNA | • Reduce the number of PCR cycles (20-35 is typical) [16].• Use less initial template DNA (e.g., 104–106 molecules) [16]. |
Experimental Protocols
Protocol 1: Basic Optimization of DMSO and Betaine for GC-Rich PCR
This protocol provides a step-by-step methodology for testing the effect of DMSO and betaine on a problematic GC-rich amplification.
Research Reagent Solutions
| Reagent | Function in the Reaction |
|---|---|
| High-Fidelity DNA Polymerase | Enzyme for DNA synthesis; some are specially formulated for GC-rich targets [29]. |
| 10X PCR Buffer | Provides optimal pH and salt conditions for the polymerase. |
| dNTP Mix (10mM) | Building blocks for new DNA strands. |
| Primers (Forward & Reverse) | Sequence-specific oligonucleotides that define the target amplicon. |
| Template DNA | The GC-rich DNA to be amplified. |
| DMSO (100%) | Additive to destabilize DNA secondary structures [30]. |
| Betaine (5M stock) | Additive to equalize DNA melting temperatures and disrupt secondary structures [30]. |
| Sterile Water | To bring the reaction to the final volume. |
Methodology:
Prepare Reaction Master Mix: Create a master mix for all reactions to minimize pipetting error. For a single 50 µL reaction, combine the following on ice:
- Sterile Water: Q.S. to 50 µL
- 10X PCR Buffer: 5 µL
- dNTP Mix (10 mM): 1 µL
- Forward Primer (20 µM): 1 µL
- Reverse Primer (20 µM): 1 µL
- DNA Polymerase: 0.5-2.5 units
- Template DNA: 1-1000 ng (optimize based on source) [6]
Aliquot and Add Additives: Aliquot the master mix into thin-walled PCR tubes. Then, add DMSO and betaine to achieve the desired final concentrations. A standard test matrix might include:
- Reaction 1: No additives (control)
- Reaction 2: 5% DMSO
- Reaction 3: 1M Betaine
- Reaction 4: 5% DMSO + 1M Betaine
Thermal Cycling: Place the tubes in a thermal cycler and run an appropriate cycling program. A suggested program, which may require optimization, is:
- Initial Denaturation: 98°C for 30 seconds to 2 minutes.
- Amplification (30-35 cycles):
- Final Extension: 72°C for 2-5 minutes.
- Hold: 4°C.
Analysis: Analyze the PCR products using agarose gel electrophoresis to assess yield, specificity, and amplicon size.
Protocol 2: Combined Additive Workflow for De Novo Gene Synthesis
This workflow is adapted from studies involving the assembly and amplification of GC-rich constructs de novo, where DMSO and betaine were critical to success [13].
Diagram 1: Experimental workflow for GC-rich gene synthesis. Based on Jensen et al. (2010) [13].
Key Experimental Steps:
- Oligodeoxynucleotide (ODN) Preparation: Design and synthesize overlapping single-stranded ODNs covering the entire GC-rich gene fragment. For ligase-based assembly (LCR), enzymatically phosphorylate the ODNs [13].
- Assembly (PCA or LCR): Perform the initial gene assembly using either Polymerase Chain Assembly (PCA) or Ligase Chain Reaction (LCR). The cited research found LCR assembly generated a more stable template for subsequent amplification. Notably, this assembly step is performed without DMSO or betaine, as their benefit was found primarily in the amplification phase [13].
- PCR Amplification with Additives: Use a small aliquot (e.g., 1 µL) of the assembled product as a template for the final amplification PCR. At this stage, include the optimized concentrations of 5% DMSO and 1M betaine in the reaction mix to efficiently amplify the full-length, GC-rich construct [13].
- Analysis and Downstream Application: Verify the product by gel electrophoresis and sequencing. The use of additives allows for the production of GC-rich constructs without the need for expensive and time-consuming purification prior to downstream applications [13].
The table below summarizes the typical concentration ranges for DMSO and betaine, providing a quick reference for experimental design.
| Additive | Common Working Concentration | Key Mechanism of Action | Key Considerations |
|---|---|---|---|
| DMSO | 2% - 10% [32] [30] | Reduces DNA secondary structure by lowering melting temperature (Tm) [30]. | Can inhibit Taq polymerase at concentrations >10% [16] [30]. |
| Betaine | 1.0 M - 2.5 M [28] [31] [32] | Equalizes Tm of GC and AT base pairs; disrupts secondary structures [13] [30]. | Use betaine monohydrate, not hydrochloride, to avoid pH shifts [30]. |
| DMSO + Betaine Combination | 5% DMSO + 1M Betaine (Recommended Starting Point) [31] [13] | Combines mechanisms to effectively denature stable GC-rich templates. | Highly compatible; no major protocol modifications needed [13]. |
A structured troubleshooting guide for researchers battling stubborn GC-rich DNA sequences in PCR.
The Core Strategy: DMSO First, Betaine on Failure
Amplification of GC-rich DNA sequences is a common challenge in molecular biology, often leading to PCR failure due to the formation of stable secondary structures that hinder polymerase progression. A strategic, sequential approach using the additives Dimethyl Sulfoxide (DMSO) and betaine can significantly improve success rates.
The recommended strategy is to include 5% DMSO by default in the initial PCR setup for GC-rich targets. If amplification fails, substitute DMSO with 1 M betaine in the subsequent attempt. Combining both additives in the same reaction generally does not provide further improvement and is not recommended. This sequential method has been demonstrated to increase the PCR success rate for challenging templates like the ITS2 DNA barcode from 42% to 100% [15].
Experimental Validation & Data
Quantitative Data on Additive Performance
The table below summarizes key experimental findings that form the evidence base for this sequential strategy:
| Study Focus | Additive(s) Tested | Optimal Concentration | PCR Success Rate / Outcome |
|---|---|---|---|
| ITS2 DNA Barcodes from Plants [15] | DMSO | 5% | 91.6% |
| Betaine | 1 M | 75% | |
| 7-deaza-dGTP | 50 µM | 33.3% | |
| Formamide | 3% | 16.6% | |
| GC-rich Disease Genes (RET, LMX1B, PHOX2B) [4] | Betaine + DMSO + 7-deaza-dGTP | 1.3 M + 5% + 50 µM | Essential for specific amplification of sequences with 67-79% GC content |
| EGFR Gene Promoter in NSCLC [18] | DMSO | 7-10% | Significant enhancement in yield and specificity |
| Glycerol | 10-20% | Significant enhancement in yield and specificity | |
| Betaine | 1-2 M | Significant enhancement in yield and specificity |
Workflow for Implementing the Sequential Strategy
The following diagram illustrates the decision-making process for using DMSO and betaine to troubleshoot a failed PCR experiment:
Detailed Experimental Protocols
Protocol 1: Initial PCR with DMSO
This protocol is adapted from plant ITS2 barcode amplification studies that achieved a 91.6% success rate with 5% DMSO [15].
Reaction Setup:
- Template DNA: 1-100 ng genomic DNA
- Primers: 0.1–1 µM each
- PCR Buffer: 1X (supplemented as per polymerase manufacturer)
- MgCl₂: 1.5–2.5 mM (adjust if not present in buffer)
- dNTPs: 200 µM each
- DMSO: 5% (v/v)
- DNA Polymerase: 0.5–2.5 units (per manufacturer's recommendation)
- Sterile Water: to final volume
Thermal Cycling Conditions:
- Initial Denaturation: 94°C for 3–5 minutes
- Amplification (25–40 cycles):
- Denaturation: 94°C for 15–30 seconds
- Annealing: Temperature specific to primer pair (e.g., 55°C) for 30 seconds
- Extension: 68°C for 1 minute per kb
- Final Extension: 68°C for 5–10 minutes
Protocol 2: Follow-up PCR with Betaine
Use this protocol when amplification with DMSO fails. This approach substitutes DMSO with betaine, based on research showing betaine can successfully amplify templates that did not respond to DMSO [15].
Reaction Setup:
- Follow Protocol 1, but make the following substitutions:
- Omit DMSO
- Add Betaine to a final concentration of 1 M
- All other components remain unchanged
Thermal Cycling Conditions:
- Identical to Protocol 1. No modification to the cycling parameters is required.
Frequently Asked Questions (FAQs)
1. Why shouldn't I use DMSO and betaine together from the start? Research specifically testing this combination for amplifying plant ITS2 barcodes found that using DMSO and betaine together did not improve the PCR success rate compared to using 5% DMSO alone. The sequential approach is more efficient and avoids unnecessary reagent interactions [15].
2. My PCR with DMSO worked but produced nonspecific bands. What should I do? Nonspecific amplification is often a sign of suboptimal stringency. Before switching to betaine, try:
- Increasing the annealing temperature in 2°C increments [33].
- Reducing the number of PCR cycles to prevent accumulation of nonspecific products [26].
- Using a hot-start DNA polymerase to minimize mispriming during reaction setup [26].
3. What if my PCR fails even with the betaine substitution? Persistent failure suggests a more complex issue. Consider these advanced troubleshooting steps:
- Re-evaluate primer design: Ensure primers are specific, have appropriate Tm, and lack secondary structures or self-complementarity [6] [26].
- Try a three-additive mixture: For extremely GC-rich targets (>75%), a powerful combination of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP has been proven essential for amplification [4]. Note that this is an exception to the general "do not combine" rule and is reserved for the most challenging cases.
- Check template quality and quantity: Ensure the DNA is intact and free of inhibitors. Verify concentration and purity using spectrophotometry or gel electrophoresis [26].
4. How do these additives actually work?
- DMSO: Interferes with the formation of DNA secondary structures (e.g., hairpins and G-quadruplexes) by disrupting hydrogen bonding and base stacking. This helps prevent polymerase pausing and strand misalignment [11].
- Betaine: Acts as a stabilizing osmolyte. It reduces the differential in melting temperature between GC-rich and AT-rich regions of the DNA by neutralizing base composition bias, thus promoting more uniform amplification [4] [11].
The Scientist's Toolkit: Essential Research Reagents
The following reagents are critical for implementing the strategies discussed in this guide.
| Reagent | Function in PCR | Typical Working Concentration |
|---|---|---|
| DMSO (Dimethyl Sulfoxide) | Disrupts secondary structures in GC-rich DNA, improving polymerase processivity and specificity [11]. | 5-10% (v/v) [15] [18] |
| Betaine (Monohydrate) | Equalizes the melting temperature (Tm) across the template, facilitating denaturation of GC-rich regions [4] [11]. | 1-1.3 M [4] [15] |
| 7-deaza-dGTP | A dGTP analog that reduces hydrogen bonding, making it easier to denature GC-rich stretches that are refractory to amplification [4]. | 50 µM (as a partial substitute for dGTP) [4] |
| High-Fidelity DNA Polymerase | Essential for obtaining accurate amplicons, especially for downstream cloning or sequencing. Prefer hot-start versions to enhance specificity [26] [34]. | As per manufacturer |
| MgClâ‚‚ / MgSOâ‚„ | Cofactor for DNA polymerase. Its concentration is critical and often requires optimization for difficult templates [6] [26]. | 1.5 - 5.0 mM |
| 6-Chloro-8-methyl-5-nitroquinoline | 6-Chloro-8-methyl-5-nitroquinoline|CAS 27527-95-3 | 6-Chloro-8-methyl-5-nitroquinoline is a versatile chemical intermediate for research applications, including pharmaceutical development. For Research Use Only. Not for human or veterinary use. |
| 2-(Benzyloxy)-4-methoxybenzoic acid | 2-(Benzyloxy)-4-methoxybenzoic acid, CAS:13618-49-0, MF:C15H14O4, MW:258.27 g/mol | Chemical Reagent |
Advanced Applications and Workflow
For highly complex de novo gene synthesis projects involving GC-rich sequences, the sequential use of additives can be integrated into a broader assembly workflow. The following diagram outlines how DMSO and betaine fit into the gene synthesis pipeline, particularly when using Ligase Chain Reaction (LCR) assembly, which has been shown to be superior for generating stable GC-rich templates [11].
The Internal Transcribed Spacer 2 (ITS2) region is a highly effective DNA barcode for species discrimination in plants, fungi, and other organisms. However, its utility has been historically limited by challenging amplification success rates, primarily due to its high GC content and propensity to form complex secondary structures that hinder polymerase progression during PCR. This technical guide addresses these limitations through optimized protocols incorporating specific PCR enhancers, enabling researchers to achieve exceptional amplification success.
Research has demonstrated that the inherent structural properties of ITS2 can reduce standard PCR success rates to as low as 42%. The implementation of a strategic protocol using the additives DMSO (dimethyl sulfoxide) and betaine can elevate this success rate to 100%, dramatically improving data yield and reliability for DNA barcoding applications [15]. This document provides a comprehensive technical support framework, including optimized protocols, troubleshooting guides, and FAQs, to assist researchers in implementing this robust methodology.
Core Experimental Protocol & Data
The following protocol and data are adapted from a study that systematically evaluated enhancers for ITS2 amplification in 50 species from 43 genera and 29 families [15].
The table below summarizes the quantitative performance of various PCR enhancers tested on 12 initially unamplifiable plant species from different families.
Table 1: Efficacy of PCR Enhancers for ITS2 Amplification
| PCR Additive | Final Concentration | PCR Success Rate | Key Observations |
|---|---|---|---|
| DMSO | 5% | 91.6% (11/12 samples) | Highest individual success rate; effectively disrupts secondary structures. |
| Betaine | 1 M | 75% (9/12 samples) | Good alternative; isostabilizing agent that equilibrates AT and GC melting temperatures. |
| 7-deaza-dGTP | 50 μM | 33.3% (4/12 samples) | Moderate success; incorporates into DNA, reducing secondary structure formation. |
| Formamide | 3% | 16.6% (2/12 samples) | Lowest success rate in this study. |
| DMSO + Betaine (Combined) | 5% + 1 M | No improvement | No synergistic effect observed; not recommended in the same reaction. |
| Default Strategy (DMSO, then Betaine) | - | 100% (50/50 samples) | Sequential use is recommended: use 5% DMSO first, substitute with 1 M betaine if failure occurs. |
Recommended Step-by-Step Protocol
Primary Reaction Setup:
- Prepare your standard PCR master mix, including primers, dNTPs, polymerase, buffer, and template DNA.
- Add DMSO to a final concentration of 5% (v/v).
- Proceed with standard thermocycling conditions appropriate for your ITS2 primer set.
Reaction Failure Triage:
- If amplification fails with 5% DMSO, repeat the PCR preparation.
- Substitute the DMSO with Betaine at a final concentration of 1 M.
- Do not combine DMSO and betaine in the same reaction tube, as this provided no improvement over DMSO alone in the foundational study [15].
Verification:
- Using this sequential strategy, a 100% success rate was achieved across a wide taxonomic range [15].
- Verify amplification by gel electrophoresis.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for ITS2 PCR Enhancement
| Reagent | Function in ITS2 PCR | Key Consideration |
|---|---|---|
| DMSO (Dimethyl Sulfoxide) | Disrupts hydrogen bonding in GC-rich regions, preventing secondary structure formation that blocks polymerase. [13] [35] | Use at 5% v/v. Higher concentrations may inhibit the polymerase. |
| Betaine (Monohydrate) | Equalizes the contribution of GC and AT base pairs to DNA melting temperature, preventing premature reannealing and stabilizing the polymerase. [13] [35] | Use at a final concentration of 1 M. |
| 7-deaza-dGTP | An analog of dGTP that is incorporated into DNA and reduces hydrogen bonding, thereby lowering the stability of secondary structures. [15] | Used at 50 μM; often less effective than DMSO or betaine. |
| Hot-Start DNA Polymerase | Reduces non-specific amplification and primer-dimer formation by requiring a heat activation step before becoming active. [26] [36] | Critical for improving specificity in challenging amplifications. |
| BSA (Bovine Serum Albumin) | Binds to and neutralizes common PCR inhibitors that may be co-extracted with DNA, such as polyphenols from plant tissues. [37] | Particularly useful for crude or difficult-to-purify templates. |
| 4-Amino-N-(3,5-dichlorophenyl)benzamide | 4-Amino-N-(3,5-dichlorophenyl)benzamide, CAS:1018501-88-6, MF:C13H10Cl2N2O, MW:281.13 g/mol | Chemical Reagent |
| 4-Methyl-3-(3-nitrobenzoyl)pyridine | 4-Methyl-3-(3-nitrobenzoyl)pyridine, CAS:1187168-01-9, MF:C13H10N2O3, MW:242.23 g/mol | Chemical Reagent |
Troubleshooting Guides and FAQs
PCR Amplification Troubleshooting
Q1: I get no amplification band for my ITS2 sample. What should I do first? A: Follow this decision flow to diagnose and resolve the issue:
The most effective first steps are to address potential inhibitors through template dilution and to include 5% DMSO in your reaction [15] [37]. If this fails, replace DMSO with 1 M betaine. Ensure your template DNA is of good quality and concentration. For difficult plant samples, adding BSA (0.1-0.5 μg/μL) can help neutralize inhibitors like polyphenols and humic acids [37].
Q2: My PCR produces a faint band or a smear. How can I improve specificity? A: Non-specific amplification, smearing, or faint bands indicate issues with reaction stringency or template quality.
- Optimize Annealing Temperature: Run a gradient PCR to determine the optimal annealing temperature for your primer-template pair. Even a 1-2°C increase can dramatically improve specificity [26] [36].
- Reduce Template Input: Too much template DNA can lead to non-specific binding and smearing. Titrate your template DNA (e.g., 1-100 ng for genomic DNA) [37] [36].
- Use a Hot-Start Polymerase: This prevents primer-dimer formation and non-specific amplification during reaction setup [26] [36].
- Check Primer Design: Ensure your primers are specific to the ITS2 region and do not form stable dimers or hairpins.
Q3: Why shouldn't I combine DMSO and betaine in the same reaction? A: The foundational study for this protocol explicitly tested the combination of 5% DMSO and 1 M betaine and found that it did not improve the PCR success rate over using 5% DMSO alone [15]. The recommended strategy is to use them sequentially, not simultaneously, to achieve the highest success rate.
Post-Amplification and Sequencing Issues
Q4: I get a clean PCR product, but my Sanger sequencing trace is messy with double peaks. What is the cause? A: Double peaks (mixed bases) in a Sanger chromatogram from a single-specimen sample suggest:
- Co-amplification of Contaminants: The sample may be contaminated with other organisms (e.g., fungal endophytes in plant tissue). Re-extract DNA from a clean sample area.
- PCR Carryover Contamination: Amplicons from previous PCRs can contaminate reagents or workspace. Use dedicated pre- and post-PCR areas, and consider implementing a dUTP/UNG carryover prevention system [37].
- Incomplete Purification: Leftover primers and dNTPs from the PCR can interfere with the sequencing reaction. Perform a thorough cleanup of the amplicon (e.g., with enzymatic Exo-SAP or bead-based kits) before sequencing [37].
Q5: For high-throughput sequencing (NGS), my ITS2 amplicon library has low diversity and poor clustering. How can I fix this? A: Amplicon libraries for loci like ITS2 have low sequence diversity in the initial cycles, which is problematic for Illumina sequencers.
- Spike-in PhiX: Add PhiX control library (e.g., 5-20%) to your final pool. This introduces nucleotide diversity during cluster generation and significantly improves data quality [37].
- Use Heterogeneity Spacers: Incorporate short, random nucleotide sequences (N-spacers) into your sequencing adapters to increase diversity in the initial sequencing cycles [37].
- Ensure Proper Cleanup: Remove any primer-dimers and free adapters with stringent bead-based size selection to prevent them from dominating the sequencing pool.
Workflow Diagram: ITS2 DNA Barcoding with Enhanced PCR
The following diagram outlines the complete workflow for successful specimen identification using the enhanced ITS2 PCR protocol, integrating morphological and genetic quality control steps.
Protocol for GC-Rich Construct Amplification in De Novo Synthesis
Amplifying GC-rich DNA constructs (GC content >65%) in de novo synthesis presents significant challenges due to secondary structure formation and mispriming, which can lead to reaction failure, nonspecific products, or truncated amplicons [38] [28] [13]. Incorporating chemical additives like Dimethyl Sulfoxide (DMSO) and betaine into PCR protocols is a well-established strategy to overcome these obstacles by destabilizing secondary structures and equilibrating the melting temperature between AT and GC base pairs [38] [4] [13]. The following table summarizes the standard concentration ranges and functions of these key additives.
Table 1: Key Additives for Enhancing GC-Rich PCR Amplification
| Additive | Final Concentration Range | Primary Function | Compatibility Notes |
|---|---|---|---|
| DMSO | 2.5% - 10% (Common: 5%) [15] [4] [39] | Disrupts secondary intermolecular and intramolecular structures [13] | >10% can inhibit Taq polymerase [40] |
| Betaine | 0.5 M - 2.5 M (Common: 1 M - 1.3 M) [6] [4] [13] | Equilibrates Tm of AT and GC base pairs; reduces nonspecific background [4] [13] | Also known as trimethylglycine |
| 7-deaza-dGTP | 50 µM (in partial replacement of dGTP) [4] | Reduces hydrogen bonding, preventing secondary structure formation [4] | Used at a 40:60 or 50:50 ratio with standard dGTP [4] [41] |
Standard Operating Procedure: PCR with DMSO and Betaine
This protocol is adapted from methods successfully used for the de novo synthesis of GC-rich gene fragments such as IGF2R and BRAF, as well as nicotinic acetylcholine receptor subunits [38] [28] [13].
Materials and Reagents
- DNA Polymerase: A robust, high-fidelity polymerase is recommended (e.g., PrimeSTAR Max, Phusion HF, or specialized GC-rich polymerases) [28] [39] [41].
- 10X Reaction Buffer: As supplied with the polymerase.
- dNTP Mix: 10 mM aqueous solution.
- Primers: Forward and reverse primers, resuspended and normalized to a working concentration (e.g., 20 µM).
- Template: Assembled construct from LCR or PCA (e.g., 1 µL of assembly reaction) [13].
- Additives: Molecular biology-grade DMSO and betaine (commercially available powder or prepared solution).
- Nuclease-Free Water.
Step-by-Step Protocol
Prepare Master Mix on Ice: For a single 50 µL reaction, combine the following components in a sterile, nuclease-free PCR tube in the order listed:
- Nuclease-Free Water: Q.S. to 50 µL final volume
- 10X PCR Buffer: 5 µL
- dNTP Mix (10 mM): 1 µL
- Forward Primer (20 µM): 1 µL
- Reverse Primer (20 µM): 1 µL
- Betaine (5 M stock): 10 µL (for a final concentration of 1 M) [13]
- DMSO: 2.5 µL (for a final concentration of 5%) [15] [13]
- DNA Template: 1–100 ng (volume variable)
- DNA Polymerase: 0.5–2.5 units (per manufacturer's recommendation)
Mix and Centrifuge: Gently pipette the entire mixture up and down at least 20 times to ensure homogeneity. Briefly centrifuge to collect all liquid at the bottom of the tube [6].
Thermal Cycling: Place the tube in a pre-heated thermal cycler and run the following program:
- Initial Denaturation: 98°C for 2–5 minutes [39]
- Amplification (25–40 cycles):
- Denaturation: 98°C for 10–30 seconds
- Annealing: Temperature optimized for your primer set (often 60–68°C) for 15–30 seconds [39]
- Extension: 72°C for 15–60 seconds per kilobase
- Final Extension: 72°C for 5–10 minutes
- Hold: 4–12°C
Post-Amplification Analysis: Analyze 5–10 µL of the PCR product by agarose gel electrophoresis to verify amplification specificity and yield.
Diagram 1: Standard PCR Workflow with Additives
FAQs and Troubleshooting Guide
How do DMSO and betaine work to improve GC-rich amplification?
GC-rich DNA sequences form strong secondary structures, such as hairpins and stem-loops, due to the three hydrogen bonds in G:C base pairs. These structures are stable and can block the progression of DNA polymerase, leading to failed or inefficient amplification [28] [13]. DMSO interferes with hydrogen bonding, disrupting these stable secondary structures and facilitating DNA strand separation [13]. Betaine, an isostabilizing agent, penetrates the DNA duplex and equalizes the contribution of GC and AT base pairs to the overall melting temperature (Tm). This prevents localized regions of very high Tm from causing incomplete denaturation and promotes uniform primer binding [4] [13]. The combined effect significantly improves polymerase processivity and product specificity.
I am using DMSO and betaine, but I still get no amplification or low yield. What should I do?
Table 2: Troubleshooting Low Yield with Additives
| Possible Cause | Recommended Solution |
|---|---|
| Suboptimal Annealing Temperature | Increase annealing temperature in 1–2°C increments to improve specificity. Use a gradient thermal cycler if available [26] [39]. |
| Insufficient Denaturation | Increase denaturation temperature to 98°C and/or extend denaturation time to ensure complete separation of GC-rich templates [26] [39]. |
| Insufficient Mg²⺠Concentration | Optimize Mg²⺠concentration (e.g., 0.5–5.0 mM). Note that additives like DMSO can affect free Mg²⺠availability [6] [26]. |
| Polymerase Inhibition | Ensure the final concentration of DMSO does not exceed 10%, as it can become inhibitory [40]. Consider using a polymerase specifically engineered for GC-rich templates [26] [28]. |
| Poor Primer Design | Redesign primers to be longer (e.g., >25 bp) with a higher Tm (>68°C). Avoid 3' ends with consecutive G or C nucleotides [26] [39]. |
Can I combine DMSO and betaine with other additives like 7-deaza-dGTP?
Yes, for extremely challenging templates (GC content >75%), a triple-additive system can be essential. Research has demonstrated that the combination of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP (replacing a portion of the standard dGTP) was required to successfully amplify a 392 bp region with 79% GC content and a 67.8% GC-rich region of the LMX1B gene [4]. 7-deaza-dGTP is incorporated by the polymerase but forms weaker hydrogen bonds than dGTP, further preventing the formation of stable secondary structures [4] [41].
Diagram 2: Logical Troubleshooting Pathway
Should DMSO and betaine be used during the assembly step (like LCR or PCA) or only during PCR amplification?
A study on the de novo synthesis of GC-rich genes found that while DMSO and betaine greatly improved target product specificity and yield during the subsequent PCR amplification step, they provided no measurable benefit when added during the Ligase Chain Reaction (LCR) or Polymerase Chain Assembly (PCA) assembly steps themselves [38] [13]. The most critical finding was that LCR assembly generated a much more stable template for amplification than PCA [13]. Therefore, the recommended workflow is to first assemble the GC-rich construct using LCR without additives, and then use a PCR protocol with DMSO and/or betaine to amplify the final assembled product.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for GC-Rich Construct Amplification
| Reagent / Kit | Specific Function | Application Note |
|---|---|---|
| High-Fidelity DNA Polymerases (e.g., PrimeSTAR GXL, Phusion HF) | Provides high processivity and fidelity for accurate amplification of difficult templates [28] [41]. | Often more tolerant of co-solvents like DMSO and betaine. |
| Specialized GC-Rich Polymerases (e.g., AccuPrime GC-Rich, Advantage GC2) | Polymerase and buffer systems specifically optimized for high GC content [40] [39]. | May include proprietary buffers that already contain Tm-equilibrating agents. |
| Molecular Biology Grade DMSO | A high-purity solvent that disrupts DNA secondary structures. | Prevents the need for glycerol-containing enzyme storage buffers that can inhibit PCR [40]. |
| Betaine (Trimethylglycine) Monohydrate | An isostabilizing agent that homogenizes the melting temperature of DNA [4] [13]. | Can be prepared as a 5M stock solution in nuclease-free water and filter-sterilized. |
| 7-deaza-2'-deoxyguanosine 5'-triphosphate | A dGTP analog that reduces hydrogen bonding in GC-rich regions [4] [41]. | Typically used as a partial substitute for dGTP in a 40:60 or 50:50 ratio. |
| Hot-Start DNA Polymerases | Prevents non-specific amplification and primer-dimer formation by requiring thermal activation [26]. | Crucial for maintaining reaction specificity, especially when using complex additive mixtures. |
Frequently Asked Questions
Can I add DMSO and betaine directly to my existing PCR protocol? Yes, both DMSO and betaine are highly compatible with standard PCR components and do not typically require additional protocol modifications [38] [13]. You can add them directly to your master mix. However, optimization of their final concentration is recommended for best results.
Do DMSO and betaine affect the activity of DNA polymerases? Some additives can influence polymerase activity. For instance, DMSO is known to reduce the activity of standard Taq polymerase, so a balance must be found between its benefits on template denaturation and its potential inhibitory effects [42]. Many modern polymerases, especially those specifically optimized for GC-rich amplification, are engineered to maintain high activity in the presence of such additives [43].
Are specialized polymerases necessary when using these additives? While DMSO and betaine can enhance PCR with many polymerase types, using a polymerase specifically designed for difficult templates often yields the best results. Polymerases like Q5 High-Fidelity or OneTaq DNA Polymerase are recommended for GC-rich targets and are often supplied with proprietary GC enhancers that may contain similar additives [43].
How do DMSO and betaine interact with Mg²âº, a critical buffer cofactor? Magnesium ion (Mg²âº) concentration is a crucial variable. It is a cofactor for DNA polymerases and facilitates primer binding by stabilizing the negative charges on DNA backbones [43] [7]. Because the reaction dynamics can shift with additives, you may need to re-optimize the Mg²⺠concentration. It is advised to test a gradient of MgClâ‚‚ (e.g., from 1.0 mM to 4.0 mM) when introducing DMSO or betaine to find the optimal concentration for your specific reaction [43] [42].
Is it better to use DMSO and betaine separately or in combination? The effectiveness of the combination can be template-specific. One study on plant DNA barcodes found that while 5% DMSO provided the highest success rate, a combination of DMSO and betaine in the same reaction did not provide further improvement [15]. Another study on a random DNA library successfully used a mix of 5% DMSO and 1 M betaine [44]. A systematic approach, testing them individually and in combination, is the most reliable strategy.
The table below summarizes the mechanisms and typical usage concentrations for DMSO and betaine, two of the most common PCR enhancers for GC-rich templates.
| Additive | Mechanism of Action | Typical Effective Concentration | Key Considerations |
|---|---|---|---|
| DMSO | Disrupts hydrogen bonding and reduces DNA secondary structure formation, lowering the melting temperature (Tm) [42]. | 2% - 10% [42]; 5% found highly effective in multiple studies [15] [44]. | Can inhibit Taq polymerase activity at higher concentrations [42]. |
| Betaine | Equalizes the contribution of GC and AT base pairs to DNA stability, acting as an isostabilizing agent. This helps prevent polymerase stalling at GC-rich regions [45]. | 1 M - 2 M [15] [18]. | Use betaine or betaine monohydrate, as betaine hydrochloride may affect reaction pH [42]. |
Experimental Protocol: Incorporating Additives for GC-Rich PCR
The following protocol is adapted from published research that successfully utilized DMSO and betaine for the de novo synthesis of GC-rich gene constructs [38] [13] and amplification of challenging DNA barcodes [15].
1. Reagent Setup: Prepare a standard PCR master mix, but omit the polymerase until after the additives have been added and mixed, if a hot-start protocol is not used. The following table outlines a sample 50 µL reaction setup.
| Component | Final Concentration/Amount | Notes |
|---|---|---|
| 10X Reaction Buffer | 1X | As supplied with the polymerase. |
| MgClâ‚‚ | 1.5 - 2.0 mM (baseline) | Requires optimization; may need increased concentration [43]. |
| dNTP Mix | 0.2 mM each | Standard concentration; ensure equimolar amounts [7]. |
| Forward Primer | 0.1 - 1 µM | Avoid high concentrations to prevent mispriming [7]. |
| Reverse Primer | 0.1 - 1 µM | Avoid high concentrations to prevent mispriming [7]. |
| Template DNA | Variable (e.g., 5-50 ng genomic DNA) | Optimize amount to avoid non-specific amplification [7]. |
| DMSO | 5% (v/v) (baseline) | Add from a high-purity, molecular biology-grade stock. |
| OR/AND Betaine | 1 M (baseline) | Add from a 5M stock solution (use betaine monohydrate) [42]. |
| DNA Polymerase | 1 - 2 units | Add last. Choose a polymerase suited for GC-rich templates [43]. |
| Nuclease-free Water | To 50 µL | - |
2. Thermal Cycling Conditions: Use the thermal cycling parameters recommended for your DNA polymerase and primer pair as a starting point. The following conditions are based on the successful amplification of the ITS2 barcode region [15]:
- Initial Denaturation: 94°C for 5 minutes.
- Amplification (35 cycles):
- Denaturation: 94°C for 30 seconds.
- Annealing: The optimal temperature must be determined empirically. Due to the Tm-lowering effect of DMSO, you may need to test a temperature gradient 2-5°C below the calculated Tm of your primers [43].
- Extension: 68°C for 45-60 seconds per kb.
- Final Extension: 68°C for 5-10 minutes.
- Hold: 4°C.
3. Optimization Strategy:
- If no product is observed: Try a lower annealing temperature gradient and/or increase the concentration of betaine (up to 2 M) [18].
- If non-specific amplification is observed: Try a higher annealing temperature gradient and/or reduce the concentration of DMSO [43].
- Systematic Testing: For critical applications, perform a matrix test, varying the concentrations of DMSO (0%, 2.5%, 5%, 7.5%) and betaine (0 M, 0.5 M, 1 M, 1.5 M) to find the optimal combination for your specific target [18].
Research Reagent Solutions
The table below lists key reagents and their roles in optimizing PCR with DMSO and betaine.
| Reagent | Function in PCR with Additives |
|---|---|
| High-Fidelity DNA Polymerase (e.g., Q5) | Ideal for long or difficult amplicons; high resistance to inhibitors and compatible with GC Enhancer [43]. |
| GC Enhancer | A proprietary buffer additive (often containing agents like DMSO and betaine) that helps inhibit secondary structure formation [43]. |
| Molecular Biology-Grade DMSO | High-purity DMSO free of contaminants that could interfere with the PCR reaction [42]. |
| Betaine (Monohydrate) | An isostabilizing agent that homogenizes the melting behavior of DNA; preferred over hydrochloride salt to avoid pH shifts [42]. |
| MgClâ‚‚ Solution | A critical cofactor for polymerase activity; its concentration often requires re-optimization when adding DMSO or betaine [43] [7]. |
Troubleshooting Workflow
This diagram outlines a logical workflow for troubleshooting PCR reactions with DMSO and betaine.
Mechanisms of Additive Compatibility
This diagram illustrates how DMSO and betaine work at the molecular level to facilitate the amplification of GC-rich DNA, highlighting their compatibility with core PCR components.
Troubleshooting PCR Failures and Fine-Tuning Additive Performance
Addressing Persistent No-Amplification or Low Yield
A Frequently Asked Question
"Despite a well-designed primer set and validated template DNA, my PCR consistently shows no amplification or very low yield on the gel. I have verified that my thermocycler programs are correct. What is the root cause of this issue, and how can I resolve it within the context of using PCR additives like DMSO and betaine?"
This common problem in the laboratory is often a symptom of the template's inherent complexity, most frequently associated with high GC content (generally over 60%) [46]. GC-rich regions form stable secondary structures, such as hairpins and stem-loops, due to the three hydrogen bonds in G-C base pairs. These structures can cause polymerase enzymes to stall, leading to premature termination, non-specific priming, and ultimately, PCR failure [4] [11]. The strategic combination of PCR additives, specifically DMSO and betaine, addresses this by destabilizing these secondary structures, providing a powerful solution to recover and optimize your assay.
The Underlying Science: How Additives Overcome GC-Rich Challenges
GC-rich DNA sequences have a higher melting temperature (Tm) and are prone to forming intrastrand secondary structures during the PCR cycling process. These structures physically block the progression of the DNA polymerase, resulting in truncated products or a complete absence of amplification [11] [46].
The combination of DMSO and betaine works through complementary mechanisms to facilitate the amplification of such difficult templates:
- Dimethyl Sulfoxide (DMSO) acts by disrupting the hydrogen bonding and hydrophobic interactions between DNA strands. It intercalates into the DNA structure, reducing its overall stability and melting temperature, which helps to keep the DNA single-stranded and accessible for primer annealing [47].
- Betaine (an amino acid analog) functions as an isostabilizing agent. It equalizes the contribution of GC and AT base pairs to the overall DNA stability. This homogenization of the melting temperature across the template prevents specific GC-rich regions from remaining double-stranded while other parts of the sequence have already denatured, thereby promoting uniform amplification and reducing the formation of secondary structures [11] [47].
When used in combination, their synergistic effect often succeeds where individual additives fail, enabling specific amplification of even extremely GC-rich targets (up to 79%) [4].
The Experimental Protocol: Combining DMSO and Betaine
The following methodology is adapted from published studies that successfully amplified GC-rich sequences from disease-related genes [4].
Materials and Reagents
- Template DNA: 100 ng genomic DNA or equivalent.
- Primers: 20 μM stock of each forward and reverse primer.
- PCR Master Mix: Includes Taq DNA polymerase, corresponding reaction buffer, and dNTPs.
- MgClâ‚‚: 25 mM stock.
- Additives:
- Betaine (Sigma-Aldrich), 5M stock solution.
- DMSO (Sigma-Aldrich), 100% stock solution.
- 7-deaza-dGTP (Roche), optional for exceptionally difficult templates.
- Nuclease-Free Water.
Step-by-Step Procedure
Prepare the Reaction Mixture Set up a 25 μL PCR reaction on ice by combining the following components in the order listed:
Component Final Concentration Volume for 1x Reaction (25 μL) Nuclease-Free Water - Q.S. to 25 μL 10X PCR Buffer 1X 2.5 μL MgCl₂ (25 mM) 2.0 - 2.5 mM 2.0 - 2.5 μL dNTP Mix (10 mM) 200 μM 0.5 μL Forward Primer (20 μM) 0.2 - 0.5 μM 0.25 - 0.625 μL Reverse Primer (20 μM) 0.2 - 0.5 μM 0.25 - 0.625 μL Betaine (5 M) 1.0 - 1.3 M 5.0 - 6.5 μL DMSO (100%) 3 - 5% (v/v) 0.75 - 1.25 μL Template DNA 100 ng Variable Taq DNA Polymerase 1.25 units 0.5 μL Note: The combined volume of Betaine and DMSO will displace a portion of the water. It is critical to calculate the final volume of water after all other components have been added. [4] [6]
Thermal Cycling Run the following cycling protocol, optimized for GC-rich targets:
- Initial Denaturation: 94°C for 3-5 minutes.
- Amplification (25-35 cycles):
- Denature: 94°C for 30 seconds.
- Anneal: 60-68°C for 30 seconds. Note: The effective annealing temperature may be lowered by DMSO; optimization might be needed. [26]
- Extend: 72°C for 1 minute per kb.
- Final Extension: 72°C for 5-10 minutes.
Product Analysis Analyze 5-10 μL of the PCR product by agarose gel electrophoresis alongside an appropriate DNA molecular weight ladder to verify the size and specificity of the amplicon.
Systematic Optimization Workflow
The following decision tree outlines a logical pathway for diagnosing and resolving persistent no-amplification issues, integrating the use of DMSO and betaine into a broader troubleshooting strategy.
Research Reagent Solutions
The table below details the key reagents discussed in this guide and their specific functions in overcoming PCR amplification challenges.
| Reagent | Function & Mechanism | Application Note |
|---|---|---|
| Betaine | Isostabilizing agent; equalizes Tm of GC and AT base pairs, reducing secondary structure formation. [11] [47] | Use at 1.0 - 1.3 M. Betaine hydrochloride can affect pH; betaine monohydrate is preferred. [4] [47] |
| DMSO | Destabilizes DNA secondary structure by reducing hydrogen bonding, thereby lowering the Tm. [47] | Use at 3 - 5% (v/v). Higher concentrations can inhibit Taq polymerase. [4] [46] |
| 7-deaza-dGTP | dGTP analog that incorporates into DNA, reducing hydrogen bonding and stability of GC-rich regions. [4] | Use at 50 μM as a partial substitute for dGTP. May require adjustment of polymerase type. [4] |
| MgClâ‚‚ | Essential cofactor for DNA polymerase activity; stabilizes the DNA double helix. [47] | Critical optimization parameter. Test a gradient from 1.0 to 4.0 mM in 0.5 mM steps. [46] |
| High-GC Polymerase | Engineered polymerases with high processivity that are less prone to stalling at secondary structures. [46] | Often supplied with proprietary "GC Enhancer" buffers. Ideal for a streamlined, one-step solution. [46] |
Key Takeaways for the Researcher
Persistent PCR failure with GC-rich templates is a solvable problem. The combination of DMSO and betaine provides a robust, synergistic chemical approach to disrupt the stable secondary structures that impede polymerization. For the most challenging targets, incorporating 7-deaza-dGTP can be the decisive factor. This troubleshooting guide provides a systematic workflow—from initial additive strategy to a powerful trio of enhancers—to help you achieve specific and high-yield amplification for your critical research and drug development projects.
Eliminating Non-Specific Products and Smeared Bands
A troubleshooting guide for researchers and drug development professionals
Why is my PCR producing smeared bands or multiple non-specific products when amplifying GC-rich sequences?
The appearance of smeared bands or multiple non-specific products is a common challenge, particularly when amplifying GC-rich templates (typically defined as sequences with >60% GC content). This occurs due to several factors rooted in the strong hydrogen bonding of GC-rich DNA:
- Stable Secondary Structures: GC-rich regions readily form hairpins and other secondary structures during the annealing step of PCR. These structures can block polymerase progression, leading to incomplete amplification and truncated products that appear as a smear or multiple bands [48] [28].
- Imperfect Primer Stringency: The high thermodynamic stability of GC-rich templates can lead to mispriming and mis-annealing, where primers bind to off-target sites with partial complementarity, resulting in the amplification of non-specific products [4] [13].
- Suboptimal Reaction Conditions: Standard PCR conditions, including magnesium concentration, polymerase type, and cycling parameters, are often inadequate for denaturing and faithfully amplifying these difficult templates [48] [49].
The Solution: Combining DMSO and Betaine in Your PCR
A highly effective strategy to overcome these issues involves the use of specific PCR additives, particularly the combination of Dimethyl Sulfoxide (DMSO) and Betaine.
- DMSO acts by disrupting the hydrogen bonding and base stacking interactions that stabilize DNA secondary structures, effectively helping to keep the DNA single-stranded and accessible [13] [48].
- Betaine is an isostabilizing agent that equalizes the thermal stability of AT and GC base pairs. It reduces the differential melting temperature across the template, preventing the formation of secondary structures and promoting uniform strand separation during PCR cycling [13] [50].
When used together, these additives create a synergistic effect that significantly improves the specificity and yield of PCR amplification for GC-rich targets [4] [50].
Experimental Protocol: Amplification with DMSO and Betaine
The following optimized protocol, adapted from published studies, provides a robust starting point for amplifying difficult GC-rich sequences [4] [50].
1. Reagent Setup Prepare a PCR master mix with the following components and final concentrations:
| Component | Final Concentration/Amount |
|---|---|
| PCR Buffer (compatible with your polymerase) | 1X |
| MgClâ‚‚ | 2.5 - 4.0 mM |
| dNTPs | 200 µM each |
| Forward Primer | 0.1 - 0.5 µM |
| Reverse Primer | 0.1 - 0.5 µM |
| DNA Template | 10 - 100 ng |
| DNA Polymerase | 1.25 units |
| Betaine | 1.0 - 1.3 M |
| DMSO | 5 - 10% (v/v) |
Note: The optimal concentration of MgClâ‚‚ may need to be titrated, as it is a critical cofactor. Higher concentrations (e.g., 4 mM) can be beneficial in some GC-rich amplifications [48] [50].
2. Thermal Cycling Conditions Use the following cycling program, which can be adjusted based on primer melting temperatures (Tm):
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 94 - 95°C | 3 - 5 min | 1 |
| Cycling | 25 - 40 | ||
| Denaturation | 94 - 95°C | 10 - 30 sec | |
| Annealing | 5 - 10°C above Tm | 30 - 60 sec | |
| Extension | 68 - 72°C | 1 min/kb | |
| Final Extension | 68 - 72°C | 5 - 10 min | 1 |
| Hold | 4 - 10°C | ∞ | 1 |
Pro Tip: Using a "touchdown" PCR approach, where the annealing temperature is gradually decreased over the first several cycles, can further enhance specificity for challenging targets [50] [51].
Optimizing Your Reaction: A Data-Driven Approach
The table below summarizes key optimization parameters and their effects, based on experimental data from multiple studies.
| Parameter | Recommendation | Effect and Rationale |
|---|---|---|
| DMSO Concentration | 5% - 10% (v/v) | Reduces secondary structure formation. Higher concentrations may inhibit polymerase activity [4] [15] [50]. |
| Betaine Concentration | 1.0 M - 1.3 M | Equalizes template melting temperatures. A study on plant ITS2 barcodes found 1M betaine provided a 75% success rate [4] [15]. |
| Magnesium (Mg²âº) | 2.5 - 4.0 mM | Critical cofactor for polymerase. Titrate in 0.5 mM increments; too little reduces yield, too much increases non-specific binding [48] [52]. |
| Polymerase Choice | Specialized enzymes (e.g., Q5, OneTaq) | Use polymerases known for high processivity or those supplied with GC enhancer buffers for superior performance on structured templates [48]. |
| Annealing Temperature | 5 - 10°C above Tm | Increases primer binding stringency, reducing off-target priming. Use a gradient to determine the optimum [48] [51]. |
The Scientist's Toolkit: Essential Research Reagents
The following table details key reagents used in this optimized PCR approach and their functions.
| Reagent | Function in GC-Rich PCR |
|---|---|
| Betaine | Isostabilizing agent that homogenizes DNA melting behavior, preventing secondary structure formation and polymerase stalling [4] [13] [50]. |
| DMSO | Polar solvent that disrupts hydrogen bonding in DNA, lowering the melting temperature and helping to denature stable GC-rich secondary structures [13] [48] [50]. |
| 7-deaza-dGTP | Guanosine analog that can be incorporated in place of dGTP; it reduces hydrogen bonding without compromising base pairing, hindering hairpin formation [4] [48]. |
| GC Enhancer | Proprietary commercial formulations (e.g., from NEB) that often contain a mixture of additives, including betaine and DMSO, to facilitate amplification of difficult templates [48]. |
| High-Fidelity DNA Polymerase | Engineered enzymes with high processivity that are less prone to stalling at complex secondary structures present in GC-rich DNA [48]. |
Experimental Workflow for Troubleshooting PCR
The diagram below outlines a logical, step-by-step workflow for diagnosing and resolving issues with non-specific bands and smearing in GC-rich PCR.
Frequently Asked Questions
Can DMSO and betaine be used together? Yes, they are highly compatible and often show a synergistic effect. A seminal study demonstrated that a combination of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP was essential for the specific amplification of several disease genes with GC content ranging from 67% to 79% [4]. However, one study on plant DNA barcodes found that while DMSO and betaine were individually highly effective, combining them did not provide further improvement [15]. It is therefore recommended to test the additives both individually and in combination for your specific template.
What should I do if adding DMSO and betaine does not work? If the problem persists, consider these additional steps:
- Titrate Mg²âº: Perform a gradient PCR with MgClâ‚‚ concentrations from 1.0 mM to 4.0 mM in 0.5 mM increments [48] [52].
- Use a Specialty Polymerase: Switch to a polymerase specifically designed for GC-rich or difficult templates, such as Q5 or OneTaq, often used with a proprietary GC enhancer solution [48].
- Optimize Primers: Re-design primers to avoid regions of high secondary structure and ensure they do not form dimers. Using longer primers can sometimes help [51].
- Reduce Template: Too much template DNA is a common cause of smearing. Try serial dilutions of your template [52] [53].
Are there any drawbacks to using DMSO or betaine? Yes, these additives must be used at optimized concentrations. Excessive DMSO (e.g., >10%) can inhibit Taq polymerase activity [50]. Similarly, while betaine is generally well-tolerated, its concentration is critical for effective isostabilization. Always include a no-additive control to accurately assess their effect on your specific PCR.
Optimizing Additive Concentration for Your Specific Template
Polymerase chain reaction (PCR) amplification of GC-rich DNA sequences presents a significant challenge in molecular biology research and diagnostic assay development. Templates with high guanine-cytosine (GC) content (>60%) form strong secondary structures due to increased hydrogen bonding, which hinders DNA polymerase progression and reduces primer annealing efficiency [54]. This technical barrier is particularly relevant for researchers working with important genomic targets such as nicotinic acetylcholine receptor subunits, tumorigenesis genes (IGF2R, BRAF), and promoter regions of clinically significant genes like EGFR [54] [13] [18].
Within this context, the strategic use of PCR additives—specifically dimethyl sulfoxide (DMSO) and betaine—provides a powerful approach to overcome these amplification challenges. This guide addresses the critical consideration of how to optimize concentrations of these additives for specific template requirements, with particular attention to the question of whether DMSO and betaine should be used separately or in combination.
FAQ: DMSO and Betaine Combination Strategies
Should I combine DMSO and betaine in the same PCR reaction?
Current research indicates that DMSO and betaine should generally be used separately rather than combined in the same reaction [15]. Studies specifically testing their combination found that it did not improve PCR success rates and sometimes inhibited amplification altogether [15] [18].
The recommended strategy is to use 5% DMSO as your default additive for GC-rich templates, substituting it with 1 M betaine only when reactions with DMSO fail [15]. This sequential approach achieved a 100% PCR success rate for amplifying the challenging ITS2 DNA barcode region across 50 plant species from 43 genera and 29 families, increasing the success rate from 42% with standard protocols [15].
What is the mechanistic basis for using these additives?
DMSO and betaine facilitate amplification of GC-rich templates through different mechanisms:
- DMSO disrupts inter- and intrastrand reannealing by weakening hydrogen bonds between base pairs, effectively destabilizing secondary structures [55] [13].
- Betaine equilibrates the differential melting temperature between AT and GC base pairings by acting as an isostabilizing agent, reducing the overall melting temperature of GC-rich regions [13].
Optimizing Additive Concentrations: Quantitative Guidance
The table below summarizes evidence-based concentration ranges for DMSO and betaine from published studies:
Table 1: Optimal Concentration Ranges for PCR Additives
| Additive | Effective Concentration Range | Most Frequently Optimal Concentration | Key Supporting Evidence |
|---|---|---|---|
| DMSO | 1–10% [6] [56] | 5–7% [15] [18] | 91.6% PCR success rate for plant ITS2 barcodes with 5% DMSO [15] |
| Betaine | 0.5–2.5 M [6] | 1 M [15] | 75% PCR success rate for plant ITS2 barcodes with 1 M betaine [15] |
Factors Influencing Concentration Optimization
The optimal concentration within these ranges depends on several factors:
- GC content percentage: Higher GC content may require concentrations at the upper end of the effective range
- Amplicon length: Longer targets may benefit from slightly lower concentrations to maintain polymerase processivity
- DNA polymerase type: Some engineered polymerases have different tolerance levels for additives
- Primer characteristics: Primers with high melting temperatures may require more additive to facilitate proper annealing
Experimental Protocol: Systematic Optimization Approach
Materials and Reagents
Table 2: Essential Research Reagent Solutions
| Reagent | Function | Example Formulation |
|---|---|---|
| High-Fidelity DNA Polymerase | Provides robust amplification with high specificity and yield for difficult templates | Phusion High-Fidelity DNA Polymerase [55] |
| 10X PCR Buffer | Maintains optimal pH and salt conditions for polymerase activity | Supplied with polymerase, often containing MgClâ‚‚ [6] |
| dNTP Mix | Building blocks for DNA synthesis | 200 μM of each dNTP (dATP, dCTP, dGTP, dTTP) [6] [55] |
| MgCl₂ or MgSO₄ | Essential cofactor for DNA polymerase activity | 1.5–4.0 mM final concentration, optimized for each template [6] [26] |
| PCR-Grade Water | Solvent for reactions, free of nucleases and contaminants | Nuclease-free, sterile-filtered water |
| DMSO Stock | Disrupts secondary structures in GC-rich templates | Molecular biology grade, stored aliquoted at room temperature |
| Betaine Stock | Equalizes melting temperatures of AT and GC base pairs | 5M stock solution, stored at -20°C |
Step-by-Step Optimization Procedure
Prepare master mix containing all standard PCR components (buffer, dNTPs, Mg²âº, polymerase, primers, template) according to manufacturer recommendations [6].
Aliquot the master mix into separate tubes for testing different additive conditions.
Add DMSO or betaine to achieve the desired final concentrations:
- Test DMSO at 3%, 5%, 7%, and 10%
- Test betaine at 0.5 M, 1.0 M, 1.5 M, and 2.0 M
- Include a no-additive control for comparison
Use appropriate cycling parameters:
Analyze results by agarose gel electrophoresis to determine which condition provides the strongest specific amplification with minimal background.
Decision Workflow for Additive Optimization
Advanced Considerations for Specific Applications
Enhancing Mutation Detection Sensitivity
For applications requiring high sensitivity in mutation detection, such as in cancer research, DMSO provides additional benefits beyond facilitating GC-rich amplification. Studies demonstrate that adding 5-10% DMSO to high-resolution melting (HRM) analysis increases mutation scanning sensitivity 2-5 fold, enabling detection of mutations with approximately 1% abundance compared to 3-10% without DMSO [55].
Troubleshooting Guide
Table 3: Troubleshooting Common Issues with PCR Additives
| Problem | Possible Cause | Solution |
|---|---|---|
| No amplification | Additive concentration too high | Test lower concentrations or reduce number of cycles |
| Non-specific bands | Additive concentration too low | Increase concentration within effective range |
| Smearing or laddering | Betaine and DMSO combination | Use additives separately rather than combined [15] |
| Inconsistent results | Poor reagent mixing | Mix reaction components thoroughly after additive addition [6] |
Optimizing DMSO and betaine concentrations for specific templates requires a systematic approach that recognizes the superior effectiveness of using these additives separately rather than in combination. The evidence-based strategy of beginning with 5% DMSO as a default and substituting with 1 M betaine when necessary provides a robust framework for overcoming the challenges of amplifying GC-rich templates. Through careful concentration optimization and adherence to the protocols outlined in this guide, researchers can significantly improve PCR success rates for even the most difficult targets, advancing progress in drug development and molecular diagnostics.
Balancing Additive Benefits with Potential Polymerase Inhibition
The amplification of difficult DNA templates, particularly those with high GC content, is a common challenge in molecular biology research and diagnostic assay development. To overcome this, additives like Dimethyl Sulfoxide (DMSO) and betaine have become essential tools in the scientist's toolkit. However, these powerful amplification facilitators present a paradox: while they can significantly improve PCR yield and specificity for challenging targets, they can also inhibit the polymerase enzyme if used improperly. This technical guide explores the balanced application of DMSO and betaine within PCR protocols, providing evidence-based strategies to maximize their benefits while minimizing potential inhibition. Within the context of optimizing reactions for GC-rich targets—frequently encountered in gene promoter regions and specific disease genes—understanding this balance becomes crucial for reliable experimental outcomes in drug development and clinical diagnostics.
Understanding the Additives: Mechanisms and Benefits
How DMSO and Betaine Facilitate Amplification
Dimethyl Sulfoxide (DMSO) is an organic solvent that enhances PCR amplification primarily by disrupting the secondary structures within DNA templates. GC-rich sequences are prone to forming stable intramolecular structures, such as hairpins and stem-loops, because G and C bases form three hydrogen bonds, compared to the two formed by A and T bases. DMSO interferes with these hydrogen bonds, effectively lowering the melting temperature (Tm) of the DNA and helping to keep the template in a single-stranded state, which is more accessible to the polymerase and primers [57] [58]. This action is particularly beneficial in preventing the polymerase from "jumping" across these secondary structures, which can lead to shortened PCR products [4]. However, it is critical to note that DMSO can also thermally destabilize DNA polymerases, and its benefits are concentration-dependent [59].
Betaine (also known as trimethylglycine) operates through a different mechanism known as "homogenization" of base stacking. It is a biologically compatible solute that equalizes the thermal stability of GC-rich and AT-rich regions within the DNA template [58]. Under standard conditions, GC-rich regions have a significantly higher melting temperature than AT-rich regions. Betaine reduces this disparity, promoting more uniform denaturation along the entire template and preventing the persistence of stable secondary structures in GC-clusters [4] [59]. Furthermore, betaine has been shown to exhibit a thermostabilizing effect on Taq DNA polymerase and enhances its tolerance to common PCR inhibitors [59].
The Synergistic Power of Combination
While effective individually, research demonstrates that DMSO and betaine can be combined for a powerful synergistic effect, especially when paired with a third additive, 7-deaza-dGTP. One study found that a combination of 1.3 M betaine, 5% DMSO, and 50 μmol/L 7-deaza-dGTP was essential for achieving specific amplification of three different disease-related genes with GC contents ranging from 67% to 79% [4] [60]. In these cases, neither additive alone, nor any two-additive combination, was sufficient to produce a clean, specific product. The combination successfully overcame the challenges of extreme GC-richness where other protocols had failed.
Table 1: Individual Functions of Common PCR Additives
| Additive | Common Working Concentration | Primary Mechanism of Action | Key Benefit |
|---|---|---|---|
| DMSO | 2–10% [58] | Disrupts hydrogen bonding, lowers DNA Tm, prevents secondary structure formation [57] | Improves specificity and yield of GC-rich amplicons [18] |
| Betaine | 1–2 M [58] | Homogenizes base-stacking stability, equalizes Tm of GC and AT regions [59] | Reduces formation of secondary structures; stabilizes polymerase [59] |
| 7-deaza-dGTP | 50 μmol/L [4] | Analog of dGTP that incorporates into DNA, disrupting Hoogsteen base-pairing | Prevents formation of secondary structures like hairpins [4] |
| Glycerol | 10–20% [18] | Stabilizes enzymes, enhances hydrophobic interactions [57] | Protects polymerase during thermal cycling; can improve yield [18] |
Quantitative Guidance and Experimental Protocols
Establishing Optimal Concentration Ranges
The efficacy and inhibitory potential of DMSO and betaine are highly concentration-dependent. The following table summarizes effective and inhibitory concentration ranges based on experimental data.
Table 2: Quantitative Effects and Optimal Ranges of DMSO and Betaine
| Additive | Effective Concentration | Reported Optimal Concentration in Combination | Signs of Inhibition / Negative Effects |
|---|---|---|---|
| DMSO | 5–10% [18] | 5% with 1.3 M Betaine & 50 µM 7-deaza-dGTP [4] | >10%: Can thermally destabilize and inhibit DNA polymerase [59]. |
| Betaine | 1–2 M [18] | 1.3 M with 5% DMSO & 50 µM 7-deaza-dGTP [4] | High Concentrations: Can decrease PCR efficiency and polymerase extension rates [59]. |
| Glycerol | 10–20% [18] | 15% with 10% DMSO [18] | >20%: Can lead to lower reaction yield and non-specific amplification [18]. |
Detailed Experimental Protocol for GC-Rich Amplification
The following protocol is adapted from a study that successfully amplified a 392-bp fragment with 79% GC content from the RET promoter region, a sequence that was refractory to standard amplification [4].
Methodology:
- Reaction Setup:
- Prepare a 25 µL total reaction volume.
- Use 1.25 units of a standard Taq DNA polymerase (e.g., from Eppendorf-5 Prime, Inc.).
- Use 1X manufacturer's reaction buffer, often supplemented with 2.5 mM MgClâ‚‚ (concentration may require optimization).
- Add 200 µM of each dNTP (dATP, dCTP, dGTP, dTTP).
- Include 50 µM 7-deaza-dGTP as a partial substitute for dGTP [4].
- Add 10 nmol of each forward and reverse primer.
- Use 100 ng of genomic DNA template.
Addition of Critical Enhancers:
- Add 1.3 M betaine (Sigma-Aldrich).
- Add 5% DMSO (Sigma-Aldrich).
- The combination of these three additives was found to be essential for specific amplification [4].
Thermal Cycling Conditions:
- Initial Denaturation: 94°C for 3–5 minutes.
- Amplification Cycles (25–40 cycles):
- Denaturation: 94°C for 30 seconds.
- Annealing: Temperature must be empirically determined. The referenced study used 60°C for 30 seconds [4]. A gradient thermal cycler is recommended for optimization.
- Extension: 72°C for 45 seconds (adjust based on amplicon length and polymerase speed).
- Final Extension: 72°C for 5–10 minutes.
Product Analysis:
- Analyze 5 µL of the PCR product by agarose gel electrophoresis.
- For confirmation, the product can be purified and sequenced using standard protocols [4].
Troubleshooting Guide and FAQs
Frequently Asked Questions
Q1: Why did my PCR reaction fail completely after adding DMSO and betaine? A: The most likely cause is excessive additive concentration, leading to polymerase inhibition. DMSO is known to thermally destabilize enzymes at high concentrations (>10%), and high levels of betaine can also reduce PCR efficiency [59]. Solution: Titrate the additives. Start with lower concentrations (e.g., 2-3% DMSO, 0.5-1 M betaine) and increase gradually if necessary. Also, ensure that you are using a high-quality, robust DNA polymerase, as some are more tolerant of additives than others.
Q2: I am getting nonspecific products even with additives. What should I do? A: While additives improve specificity for the intended target, they can sometimes reduce the overall stringency of primer annealing. Solution: Re-optimize the annealing temperature. Increase it in 1–2°C increments to enhance stringency [26] [58]. Also, verify your primer design to ensure specificity and avoid self-complementarity.
Q3: How do I know if my PCR failure is due to additive inhibition or another issue? A: Run a systematic control experiment:
- Positive Control: A known, easy-to-amplify template with the same primers and additives.
- Additive Titration: A set of reactions with your target template and a gradient of additive concentrations (e.g., 0%, 2%, 5%, 10% DMSO).
- No-Additive Control: Your target template in a standard reaction without any additives. This will help you distinguish between inhibition, a failed reaction setup, and a template that simply requires enhancers to amplify.
Q4: Can I simply use a more robust polymerase instead of these additives? A: Yes, this is a valid strategy. Many modern, high-fidelity DNA polymerases are specifically engineered for high processivity and resilience to difficult templates and inhibitors [57] [26]. They often come with proprietary buffers that may already include stabilizing agents. However, for extremely challenging GC-rich targets, a combination of a specialized polymerase and the DMSO/betaine mixture may still be necessary [4] [61].
Troubleshooting Flowchart
The following diagram outlines a logical workflow for diagnosing and addressing issues related to PCR additive inhibition.
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Reagents for PCR Enhancement and Inhibition Management
| Reagent / Kit | Function / Application | Example Use Case |
|---|---|---|
| PowerClean DNA Clean-Up Kit | Effective removal of a wide range of PCR inhibitors (e.g., humic acid, collagen, hematin) from DNA extracts [62]. | Purifying DNA from forensic, environmental, or plant samples known to contain inhibitors. |
| High-Fidelity DNA Polymerases (e.g., Q5, Phusion) | Engineered for high specificity and processivity; often more tolerant of complex templates and buffer additives [61]. | Amplifying GC-rich sequences for cloning or sequencing, where high fidelity is critical. |
| Hot-Start DNA Polymerases | Remains inactive at room temperature, preventing non-specific amplification and primer-dimer formation before thermal cycling [26]. | Improving specificity in all PCRs, especially when using complex primer sets or suboptimal conditions. |
| 7-deaza-dGTP | A dGTP analog that incorporates into DNA and prevents the formation of secondary structures by disrupting Hoogsteen base-pairing [4]. | Essential component for amplifying extremely GC-rich sequences (>75%) when combined with DMSO and betaine. |
| BSA (Bovine Serum Albumin) | Binds to inhibitors commonly found in biological samples (e.g., phenolics, humic acid), neutralizing their effects [57]. | Alleviating inhibition when amplifying from complex samples like blood, plasma, or plant tissues. |
FAQs on Template and Primer Troubleshooting
What are the primary causes of PCR failure related to the DNA template?
PCR failure can often be traced to issues with template DNA quality, quantity, or complexity.
- Poor Template Integrity: Degraded DNA can lead to smears on gels or high background. Always minimize DNA shearing during isolation and store DNA in molecular-grade water or TE buffer (pH 8.0) to prevent nuclease degradation [26].
- Insufficient Template Purity: Residual contaminants like phenol, EDTA, or salts can inhibit DNA polymerases. Re-purify your template DNA, for instance by ethanol precipitation, to remove these inhibitors [26].
- Incorrect Template Quantity: Using too much DNA can cause nonspecific amplification, while too little can result in low or no yield [26] [7]. The optimal amount depends on the DNA source:
- Plasmid DNA: 0.1–1 ng per 50 µL reaction.
- Genomic DNA (gDNA): 5–50 ng per 50 µL reaction.
- Complex Template Sequences: GC-rich templates (with a GC content of 60% or higher) are a common challenge. Their strong secondary structures (like hairpins) can block polymerase progression and resist denaturation [63].
How can I tell if my primers are poorly designed, and what are the key design rules?
Poor primer design is a major cause of nonspecific products, primer-dimer formation, or no amplification. Adhere to the following design principles [6] [7]:
- Length: 15–30 nucleotides.
- Melting Temperature (Tm): 55–70°C, with the Tms of the forward and reverse primer within 5°C of each other.
- GC Content: 40–60%, with a uniform distribution of G and C bases.
- 3' End: Should end with a C or G to promote "anchoring," but avoid runs of more than three G or C bases.
- Specificity: Avoid self-complementarity (hairpins), complementarity to the other primer (primer-dimer), and direct repeats.
Use online tools like NCBI Primer-BLAST or Primer3 to check for specificity and calculate accurate Tm values [6].
My PCR works with a control template but fails with my target GC-rich sequence. What should I check first?
When amplifying GC-rich sequences, standard PCR conditions often fail. Your systematic approach should include [26] [63]:
- Polymerase Choice: Standard Taq may stall at secondary structures. Switch to a polymerase specifically engineered for GC-rich and difficult templates, such as Q5 or OneTaq DNA Polymerase, which are often supplied with a proprietary GC Enhancer [63].
- PCR Additives: Incorporate additives that disrupt secondary structures. A combination of DMSO and betaine has been proven essential for amplifying sequences with GC content from 67% to 79% [4] [11].
- Thermal Cycling Conditions: Increase the denaturation temperature or time to ensure full separation of the stubborn double-stranded DNA [26].
What is the role of DMSO and betaine in amplifying difficult templates?
DMSO and betaine are isostabilizing agents that greatly improve the amplification of GC-rich constructs [11] [13].
- DMSO disrupts inter- and intrastrand secondary structure formation (e.g., hairpins) by interfering with hydrogen bonding, which helps keep the DNA single-stranded and accessible [11].
- Betaine equilibrates the differential stability between AT and GC base pairs. It reduces the effective melting temperature of GC-rich regions, making it easier to denature the template during PCR cycling [11].
When used in combination, they can be essential for achieving specific amplification of extremely challenging targets [4].
Experimental Protocols for Optimizing PCR with Additives
Protocol 1: Initial Setup with DMSO and Betaine
This protocol is adapted from a study that successfully amplified GC-rich disease genes using a combination of three additives [4].
Materials:
- Taq DNA Polymerase (e.g., from Eppendorf or Applied Biosystems)
- 10X PCR Buffer (with or without MgClâ‚‚)
- dNTP Mix (10 mM each)
- Forward and Reverse Primers (20 µM each)
- Template DNA (e.g., 100 ng genomic DNA)
- Betaine (5 M stock solution)
- DMSO
- 7-deaza-dGTP (optional, for extremely difficult templates)
- Sterile distilled water
Method:
- Prepare a 50 µL PCR master mix on ice in the following order:
- 34.5 µL Sterile Water
- 5.0 µL 10X PCR Buffer
- 1.0 µL dNTP Mix (10 mM)
- 1.0 µL Forward Primer (20 µM)
- 1.0 µL Reverse Primer (20 µM)
- 0.5 µL Taq DNA Polymerase (e.g., 1.25 units)
- Additives:
- 13.0 µL Betaine (5 M stock to achieve ~1.3 M final concentration)
- 2.5 µL DMSO (to achieve 5% final concentration)
- 1.0 µL 7-deaza-dGTP (50 µM final concentration; optional)
- 1.0 µL Template DNA
- Mix gently by pipetting and centrifuge briefly.
- Run the following thermal cycling protocol [4]:
- Initial Denaturation: 94°C for 3–5 minutes
- 25–35 Cycles:
- Denature: 94°C for 10–30 seconds
- Anneal: 55–60°C for 30 seconds
- Extend: 68°C for 1 minute (adjust based on product length)
- Final Extension: 68°C for 5 minutes
Protocol 2: Optimization of Additive Concentrations
If the initial setup does not yield optimal results, systematically optimize the concentration of DMSO and betaine. The table below summarizes typical working concentrations for various additives [6] [63].
Table 1: Common PCR Additives and Their Usage
| Additive | Final Concentration Range | Primary Function |
|---|---|---|
| DMSO | 1–10% | Disrupts secondary structures, reduces DNA melting temperature [6] [11]. |
| Betaine | 0.5 M – 2.5 M | Equalizes the stability of AT and GC base pairs, reduces melting temperature [6] [11]. |
| Formamide | 1.25–10% | Increases primer stringency, improving specificity [6] [63]. |
| 7-deaza-dGTP | 50 µM (can be used to partially replace dGTP) | Analog of dGTP that base-pairs with dCMP but disrupts Hoogsteen base-pairing, preventing hairpin formation [4]. |
| GC Enhancer | As per manufacturer's instructions | Proprietary blends (often containing agents like DMSO and betaine) optimized for specific polymerases [63]. |
Optimization Workflow:
- Keep one additive constant: Start with 5% DMSO and test a betaine gradient (0.5 M, 1.0 M, 1.5 M, 2.0 M).
- Evaluate results on an agarose gel for specificity and yield.
- Refine further: Using the best betaine concentration, test a DMSO gradient (2%, 5%, 7%, 10%).
- Consider also adjusting the annealing temperature in 1–2°C increments, as additives can lower the effective Tm of the primers [26].
Research Reagent Solutions
The following table details key reagents used in troubleshooting PCR for GC-rich templates.
Table 2: Essential Reagents for GC-Rich PCR Troubleshooting
| Reagent | Function in PCR | Considerations for GC-Rich Targets |
|---|---|---|
| High-Performance DNA Polymerase (e.g., Q5, OneTaq) | Catalyzes DNA synthesis. | Engineered for high processivity and affinity to overcome polymerase stalling at secondary structures [26] [63]. |
| Betaine | Isostabilizing agent. | Final concentration of 1.3 M is commonly used; often more effective when combined with DMSO [4]. |
| DMSO | Secondary structure destabilizer. | Final concentration of 5% is commonly used; can enhance the effect of betaine [4] [11]. |
| 7-deaza-dGTP | dGTP analog. | Used at 50 µM final concentration; note that PCR products containing it stain poorly with ethidium bromide [4] [63]. |
| MgCl₂ | Essential cofactor for polymerase activity. | Concentration may require optimization (test 0.5 mM increments from 1.0–4.0 mM); too much causes nonspecific binding, too little reduces yield [6] [63]. |
| GC Enhancer | Proprietary additive blend. | Often provided with specialized polymerases; a convenient option as it is pre-optimized for the enzyme [63]. |
Workflow Diagrams
PCR Troubleshooting Logic
Additive Optimization Path
Evidence and Comparisons: Validating Efficacy Against Other Enhancers
Troubleshooting Guides & FAQs
What is the primary challenge when amplifying GC-rich DNA sequences, and how can it be solved?
GC-rich DNA templates (typically >60% GC content) are prone to forming stable secondary structures and intramolecular hairpins. This can cause the DNA polymerase to stall, resulting in poor amplification yield, non-specific products, or complete PCR failure [4] [11] [64]. A powerful solution is to use PCR additives, also known as enhancers or co-solvents, which help denature these stubborn structures. Common additives include DMSO, betaine, formamide, and 7-deaza-dGTP [4] [6] [18].
When should I use a combination of DMSO and betaine over other additives?
The combination of DMSO and betaine is particularly effective for the de novo synthesis of GC-rich constructs and for uniformly amplifying complex DNA libraries, such as those used in aptamer selection [11] [44]. While formamide can also improve specificity [6], and 7-deaza-dGTP can be essential for the most challenging targets [4], the DMSO/betaine mixture is often a good first choice due to its broad efficacy, low cost, and high compatibility with standard PCR protocols [11] [13].
A combination of three additives was needed for my experiment. Is this common?
For extremely challenging templates with GC content exceeding 70%, a triple-additive mixture can be indispensable. One study found that a combination of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP was essential to achieve specific amplification of several disease genes with GC content ranging from 67% to 79% [4]. In these cases, two-additive combinations either failed or still produced non-specific bands.
My PCR product is a smear or has multiple bands when amplifying a GC-rich target. What should I do?
This is a classic sign of non-specific amplification and secondary structure formation. First, ensure your primer design is optimal. Then, incorporate DMSO and betaine into your reaction. One study showed that this combination drastically reduced nonspecific background and was crucial for obtaining a clean, specific product [4]. You should also optimize your thermal cycling conditions, specifically by using a shorter annealing time (3-6 seconds) for GC-rich templates, as this can minimize mispriming [64].
Table 1: Performance Comparison of Common PCR Additives for GC-Rich Templates
| Additive | Common Working Concentration | Key Mechanism of Action | Reported Efficacy (Example Studies) |
|---|---|---|---|
| DMSO & Betaine Combination | 5-10% DMSO + 0.5 M - 1.3 M Betaine | Betaine equilibrates Tm; DMSO disrupts secondary structures [11] [64]. | Essential for specific amplification in de novo gene synthesis [11] [13]. Improved uniform amplification of random DNA library [44]. |
| Formamide | 1.25 - 10% [6] | Acts as a denaturant to prevent secondary structure formation [6]. | Can dramatically improve the specificity of PCR [6]. |
| 7-deaza-dGTP | 50 µM (with standard dGTP) [4] | Replaces dGTP, reducing hydrogen bonding and melting temperature of GC pairs [4]. | Alone, insufficient for 79% GC target; essential only in triple mixture with DMSO and betaine [4]. |
| DMSO, Betaine, & 7-deaza-dGTP | 5% DMSO + 1.3 M Betaine + 50 µM 7-deaza-dGTP | Combined effect of all three mechanisms for maximum disruption of stable structures [4]. | Essential to achieve specific amplification of sequences with 67-79% GC content [4]. |
Table 2: Optimized Protocol for Challenging GC-Rich Amplification
This protocol is adapted from a study that successfully amplified a 392 bp fragment with 79% GC content [4].
| Reaction Component | Final Concentration / Amount |
|---|---|
| DNA Polymerase | 1.25 units (e.g., Taq or Gold Taq) |
| PCR Buffer | 1X (supplemented with 2.0 - 2.5 mM MgClâ‚‚) |
| dNTPs | 200 µM of each dATP, dCTP, dTTP |
| dGTP / 7-deaza-dGTP | 150 µM dGTP + 50 µM 7-deaza-dGTP |
| Primers (Forward & Reverse) | 10 nmol each (typically 0.1-1 µM) |
| Template DNA | 100 ng genomic DNA |
| Betaine | 1.3 M |
| DMSO | 5% (v/v) |
| Sterile Water | To final volume (e.g., 25 µl) |
Experimental Protocol: Amplification of a GC-Rich Template Using a Triple-Additive Mixture
Method
- Reaction Setup: Prepare a master mix on ice containing all the components listed in Table 2. Gently mix the reagents by pipetting up and down at least 20 times to ensure homogeneity, as Taq polymerase is often stored in glycerol [6].
- Thermal Cycling:
- Initial Denaturation: 94°C for 3-5 minutes.
- Amplification Cycles (25-40 cycles):
- Final Extension: 72°C for 5-10 minutes.
- Product Analysis: Analyze 5 µL of the PCR product by agarose gel electrophoresis to check for specificity and yield [4].
Mechanism of Action & Experimental Workflow
Research Reagent Solutions
Table 3: Essential Reagents for PCR of GC-Rich DNA
| Reagent / Solution | Function / Explanation |
|---|---|
| High-Quality Thermostable DNA Polymerase | Essential for efficient amplification. Hot-start polymerases are recommended to increase specificity by preventing non-specific amplification during reaction setup [26]. |
| Betaine (Molecular Biology Grade) | An isostabilizing agent that equalizes the contribution of AT and GC base pairs to the template's melting temperature (Tm), facilitating the denaturation of stable structures [4] [64]. |
| Dimethyl Sulfoxide (DMSO) | A polar solvent that disrupts hydrogen bonding and inter-/intrastrand base pairing, helping to unwind DNA secondary structures [11] [64]. |
| 7-deaza-2'-deoxyguanosine-5'-triphosphate (7-deaza-dGTP) | An analog of dGTP that is often used partially to replace dGTP. It lacks a nitrogen atom involved in Hoogsteen base pairing, which helps prevent the formation of stable secondary structures [4]. |
| Optimized Mg²⺠Solution | Mg²⺠concentration is critical for polymerase activity and primer annealing. Its concentration often needs optimization, especially when additives are present, as they can affect its availability [6] [26]. |
Comparative Analysis in Demanding Real-World Scenarios (e.g., rAAV ITR Sequencing)
Frequently Asked Questions (FAQs)
Q1: Why is amplifying GC-rich sequences like rAAV ITRs so challenging for PCR? GC-rich DNA sequences form stable secondary structures, such as hairpins, due to the three hydrogen bonds between Guanine (G) and Cytosine (C). These structures cause the polymerase to pause or fall off, leading to truncated PCR products, mispriming, and ultimately, amplification failure or nonspecific results [13] [65].
Q2: How do DMSO and Betaine work to improve PCR of difficult templates? DMSO and Betaine are PCR additives that act through different but complementary mechanisms to disrupt DNA secondary structures.
- DMSO: Interferes with DNA secondary structure formation by disrupting hydrogen bonding and base stacking interactions. This helps prevent the template from forming stable hairpins and other complex structures [13].
- Betaine: An isostabilizing agent that reduces the differential melting temperature (Tm) between AT-rich and GC-rich regions. It equilibrates the Tm across the DNA fragment, making the entire sequence melt at a more uniform temperature during PCR cycling, which facilitates primer annealing and polymerase progression [13].
Q3: What is the recommended combination and concentration for DMSO and Betaine? Research indicates that a combination of 1.3 M Betaine and 5% DMSO (v/v) is highly effective for amplifying GC-rich constructs [4] [13]. This specific combination has been shown to be essential for achieving clean, specific amplification of targets with GC content exceeding 67% [4].
Q4: Can I use this additive combination with any DNA polymerase? While DMSO and Betaine are compatible with many common DNA polymerases, it is crucial to consult the manufacturer's instructions for your specific enzyme. Some polymerases are supplied with proprietary buffers that may already contain enhancers, and the addition of DMSO/Betaine could affect enzyme activity. Always include a control reaction without additives for comparison [26] [66].
Q5: My PCR still shows smearing or multiple bands after adding DMSO and Betaine. What should I do? The presence of smearing suggests that nonspecific amplification is still occurring. You can try the following steps to increase stringency:
- Increase the annealing temperature in increments of 2°C [66] [65].
- Use a hot-start DNA polymerase to prevent primer-dimer formation and mispriming during reaction setup [26] [66].
- Reduce the number of PCR cycles to minimize the accumulation of nonspecific products [26].
- Verify your primer design to ensure specificity for the target sequence [66].
Troubleshooting Guide
The table below outlines common problems, their potential causes, and solutions when working with challenging PCR templates like rAAV ITRs.
| Observation | Possible Cause | Recommended Solution |
|---|---|---|
| No PCR Product | Excessively high annealing temperature; poor primer design; severe secondary structures | Lower annealing temperature in 2°C increments [65]; redesign primers; use 1.3 M Betaine + 5% DMSO [4] [13]; increase extension time [26]. |
| Faint or Low Yield | Insufficient number of cycles; low primer concentration; inefficient denaturation | Increase cycle number (up to 40 cycles) [65]; optimize primer concentration (0.1-1 µM) [26]; increase denaturation time/temperature [26]. |
| Multiple Bands or Smearing | Low annealing temperature; primer dimers or nonspecific binding; excess template | Increase annealing temperature [66] [65]; use hot-start polymerase [26] [66]; reduce template amount by 2-5 fold [65]; apply touchdown PCR [65]. |
| PCR Errors/Incorrect Sequence | Low-fidelity polymerase; unbalanced dNTPs; excess Mg2+; overcycling | Use a high-fidelity polymerase [66]; ensure equimolar dNTP concentrations [26] [66]; optimize Mg2+ concentration [66]; reduce number of cycles [66]. |
Detailed Experimental Protocol: Amplification of GC-rich rAAV ITR Sequences
This protocol is adapted from established methods for de novo synthesis of GC-rich genes and amplification of demanding targets [4] [13].
Reagent Setup
- 10x PCR Buffer: As supplied with the DNA polymerase.
- dNTP Mix: 10 mM of each dNTP.
- Forward and Reverse Primers: 10 µM each, resuspended in nuclease-free water.
- Template DNA: < 100 ng of rAAV vector or plasmid DNA [65].
- High-Fidelity DNA Polymerase: e.g., Q5 or Phusion [66].
- PCR Additives:
- 5 M Betaine stock: Prepare in nuclease-free water and filter sterilize.
- 100% DMSO: Molecular biology grade.
PCR Reaction Assembly
Prepare a 50 µL reaction mixture on ice as follows. Include a control reaction without additives for comparison.
| Component | Volume (µL) | Final Concentration |
|---|---|---|
| Nuclease-free Water | To 50 µL | - |
| 10x PCR Buffer | 5 | 1x |
| 5 M Betaine Stock | 13 | 1.3 M |
| 100% DMSO | 2.5 | 5% |
| 10 mM dNTP Mix | 1 | 200 µM each |
| 10 µM Forward Primer | 2.5 | 0.5 µM |
| 10 µM Reverse Primer | 2.5 | 0.5 µM |
| Template DNA | X | Variable |
| DNA Polymerase | Y | As per mfr. |
Thermal Cycling Conditions
Use the following cycling parameters, optimized for GC-rich templates [4] [13]:
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Initial Denaturation | 98°C | 2-5 minutes | 1 |
| DenaturationAnnealingExtension | 98°C60-68°C*72°C | 10-30 seconds15-30 seconds30-60 sec/kb | 30-40 |
| Final Extension | 72°C | 5-10 minutes | 1 |
| Hold | 4°C | ∞ | 1 |
*The optimal annealing temperature must be determined empirically. Start with a gradient PCR 5°C below the lower primer Tm [66].
Research Reagent Solutions
The table below details key reagents used in the featured protocol and their critical functions.
| Reagent | Function/Explanation |
|---|---|
| Betaine | Isostabilizing agent that homogenizes melting temperatures across GC-rich regions, preventing polymerase stalling [4] [13]. |
| DMSO | Disrupts hydrogen bonding in DNA secondary structures (e.g., hairpins), facilitating strand separation during denaturation [13]. |
| High-Fidelity DNA Polymerase | Engineered enzymes with proofreading (3'→5' exonuclease) activity that significantly reduce error rates during amplification, crucial for downstream applications [66]. |
| dNTP Mix | The building blocks for DNA synthesis. Using a fresh, balanced equimolar mix is critical to prevent misincorporation and ensure high yield [26] [66]. |
Experimental Workflow and Mechanism of Action
The following diagram illustrates the key steps in the optimized PCR protocol for challenging sequences.
Diagram Title: Workflow for GC-Rich PCR
This diagram outlines the mechanistic action of DMSO and Betaine in neutralizing the challenges posed by GC-rich DNA during PCR.
Diagram Title: Mechanism of DMSO and Betaine
Limitations and Scenarios Where DMSO and Betaine Are Less Effective
Troubleshooting Guides
Guide 1: Addressing Failed Amplification with Additives
Problem: Even with the use of DMSO, betaine, or their combination, the PCR amplification fails or shows very poor yield.
Explanation: Additives are not a universal remedy. Their effectiveness is highly dependent on the specific template and reaction conditions. The specific DNA sequence, the type of DNA polymerase used, and the precise concentrations of all reaction components play a critical role.
Solutions:
- Re-optimize Additive Concentration: Systematically test a range of concentrations for a single additive. For DMSO, try 2% to 10%; for betaine, test 0.5 M to 2 M [18] [67].
- Evaluate Polymerase and Buffer: Switch to a DNA polymerase specifically engineered for amplifying GC-rich or difficult templates. Re-optimize the MgClâ‚‚ concentration, as it is a critical cofactor and its optimal level can shift when additives are introduced [7].
- Redesign Primers: Re-evaluate your primer design. Ensure they have appropriate melting temperatures (55–70°C), are within 5°C of each other, and have a GC content of 40–60% without stable secondary structures [7].
Guide 2: Managing Non-specific Amplification and Smearing
Problem: The PCR reaction produces multiple non-specific bands or a smear on the gel, despite the presence of DMSO or betaine.
Explanation: While these additives can improve specificity, using them at incorrect concentrations can have the opposite effect. High concentrations of DMSO can reduce Taq polymerase activity and potentially destabilize primer binding, leading to mis-priming [67]. High primer concentrations are also a common source of non-specific amplification.
Solutions:
- Titrate Additive Down: High concentrations of DMSO (e.g., >10%) can be inhibitory. Gradually reduce the concentration of the additive [67].
- Lower Primer Concentration: Reduce the primer concentration to the range of 0.1–0.5 µM to minimize the chance of mis-priming [7].
- Increase Annealing Temperature: Use a thermal gradient PCR to determine a higher, more stringent annealing temperature. Betaine can help maintain DNA duplex stability at higher temperatures, aiding this approach [67].
Guide 3: When Additive Combinations Are Detrimental
Problem: Combining DMSO and betaine in the same reaction does not improve amplification and sometimes makes it worse.
Explanation: Synergistic effects are not guaranteed. Some studies report that combining DMSO and betaine did not improve PCR success rates and, in some cases, failed to amplify the target altogether, whereas either additive used alone was successful [15] [68]. The combined effect may over-stabilize or destabilize the DNA duplex in a manner that is counterproductive for a specific template.
Solutions:
- Use Additives Sequentially, Not in Combination: Adopt a strategy of using one additive at a time. A recommended protocol is to include 5% DMSO by default and substitute it with 1 M betaine only if the reaction with DMSO fails [15] [68].
- Test a Three-Additive Mixture (For Extreme Cases): For exceptionally challenging, GC-rich targets (GC content >70%), a combination of 1.3 M betaine, 5% DMSO, and 50 µM 7-deaza-dGTP has been shown to be essential for specific amplification where two-additive mixes failed [4].
Frequently Asked Questions (FAQs)
FAQ 1: At what concentration does DMSO become inhibitory to PCR? While DMSO at 5-10% can be beneficial, higher concentrations become increasingly inhibitory. One study found that DMSO concentrations at 7% and 10% worked well, but other combinations at high concentrations blocked amplification entirely [18]. Concentrations above 20% are used in non-PCR contexts to denature DNA, which would be detrimental to a standard PCR [14].
FAQ 2: Can betaine and DMSO be used for templates other than genomic DNA? Yes. Both additives have been successfully used to amplify difficult targets in various applications, including the de novo synthesis of GC-rich gene constructs [13] and the amplification of DNA barcodes (ITS2) from plants [15]. Their primary function is to disrupt secondary structures, which is a common problem across different DNA template sources.
FAQ 3: Why might my GC-rich PCR work with betaine but not with DMSO? DMSO and betaine operate through distinct mechanisms. DMSO interacts with water molecules to reduce DNA secondary structure stability and lower melting temperature [67]. Betaine acts as an isostabilizer, reducing the difference in stability between GC and AT base pairs and preventing the formation of secondary structures [67]. Your specific template and its propensity to form certain secondary structures may be more effectively resolved by one mechanism over the other.
FAQ 4: Do DMSO and betaine affect DNA polymerase fidelity? The search results do not provide specific data on the direct impact of DMSO or betaine on the error rate of DNA polymerases. The primary focus of the available literature is on their role in improving amplification yield and specificity by mitigating secondary structures.
Table 1: Effects of DMSO and Betaine on DNA and PCR Performance
| Parameter | Effect of DMSO | Effect of Betaine | Experimental Context |
|---|---|---|---|
| Optimal Concentration | 2-10% [18] [67] | 1-2 M [15] [18] | PCR amplification |
| Inhibitory Concentration | >10% (varies by system) [18] | Concentrations >2 M may be inhibitory [18] | PCR amplification |
| DNA Persistence Length | Decreases by 0.43% per %-DMSO (up to 20%) [14] | Information not available in results | Single-molecule DNA mechanics |
| PCR Success Rate | 91.6% (ITS2 plant barcodes) [15] | 75% (ITS2 plant barcodes) [15] | Plant DNA barcoding |
| Combination Efficacy | Not recommended as a first-line combination [15] [68] | Not recommended as a first-line combination [15] [68] | Standard GC-rich PCR |
Table 2: Alternative Additives and Their Applications
| Additive | Common Concentration | Primary Mechanism | Example Use Case |
|---|---|---|---|
| Formamide | 1-5% [67] | Reduces DNA Tm and destabilizes secondary structures [67] | Improved specificity in GC-rich amplification [15] |
| 7-deaza-dGTP | 50 µM [4] | Replaces dGTP, reducing hydrogen bonding in GC-rich regions [4] | Essential for amplifying sequences with >70% GC content when combined with DMSO/betaine [4] |
| Glycerol | 10-20% [18] | Stabilizes polymerase; can destabilize DNA duplex [18] | Enhanced yield and specificity for EGFR promoter [18] |
Experimental Protocols
Protocol 1: Systematic Optimization of a Single Additive
Method: This protocol is adapted from studies that tested multiple additives and concentrations to amplify challenging GC-rich regions like the EGFR promoter and plant ITS2 barcodes [18] [15] [68].
- Prepare a Master Mix: Create a standard PCR master mix containing all core components (buffer, dNTPs, primers, polymerase, template).
- Aliquot and Supplement: Aliquot the master mix into multiple tubes. To each tube, add a different volume of the additive (DMSO, betaine, or glycerol) to create a concentration gradient.
- DMSO: 0%, 2%, 5%, 7%, 10% (v/v)
- Betaine: 0 M, 0.5 M, 1.0 M, 1.5 M, 2.0 M
- Amplify: Run the PCR using your standard cycling parameters.
- Analyze: Resolve the PCR products on an agarose gel. Identify the concentration that gives the strongest specific band with the least background.
Protocol 2: Three-Additive Mixture for Highly Refractory Targets
Method: This protocol is based on work that successfully amplified genomic sequences with GC content ranging from 67% to 79% [4].
- Reaction Setup: In a total volume of 25 µL, combine:
- 1X PCR buffer (supplemented with 2.5 mM MgClâ‚‚)
- 200 µM of each dNTP
- 10 pmol of each primer
- 100 ng of genomic DNA
- 1.25 units of Taq DNA polymerase
- Add Additives: Include the following additives in the reaction mix:
- 1.3 M betaine
- 5% DMSO
- 50 µM 7-deaza-dGTP (Note: This is a partial substitution for dGTP)
- Thermal Cycling: Perform amplification with an initial denaturation at 94°C for 5 minutes, followed by 30-40 cycles of 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 45-60 seconds, with a final extension at 72°C for 5 minutes.
Workflow and Relationship Diagrams
Diagram 1: Additive Troubleshooting Workflow
Research Reagent Solutions
Table 3: Essential Materials for PCR Additive Experiments
| Reagent / Material | Function / Explanation | Example Use Case |
|---|---|---|
| DMSO (Dimethyl Sulfoxide) | Polar aprotic solvent; reduces DNA secondary structure by lowering melting temperature (Tm) [14] [67]. | Standard aid for GC-rich templates at 5-10% concentration [15] [18]. |
| Betaine (Monohydrate) | Osmoprotectant; acts as isostabilizer to equalize Tm of AT and GC base pairs, preventing secondary structure formation [67]. | Used at 1-2 M for difficult templates; can be effective where DMSO fails [15] [68]. |
| 7-deaza-dGTP | Modified nucleotide; reduces hydrogen bonding in GC-rich regions by replacing nitrogen at position 7 of the guanine ring [4]. | Critical component in 3-additive mix for extremely GC-rich targets (>70% GC) [4]. |
| High-Fidelity DNA Polymerase | Engineered enzymes with better processivity and resistance to inhibitors present in complex templates or additive mixes. | Essential for long or difficult amplifications; requires buffer re-optimization with additives [7]. |
| GC-Rich Control Template | A known, challenging DNA template used as a positive control to validate additive efficacy and reaction setup. | Validates protocol when troubleshooting new templates or reagent batches. |
In polymerase chain reaction (PCR) research, the amplification of GC-rich DNA sequences presents a significant challenge due to the formation of stable secondary structures that impede polymerase activity. Within this context, the strategic combination of dimethyl sulfoxide (DMSO) and betaine has emerged as a powerful methodological approach to disrupt these structures and facilitate successful amplification of previously refractory templates. This technical support center provides detailed troubleshooting guides and experimental protocols for researchers implementing these oligonucleotide 'disruptors' in their experimental workflows.
How do DMSO and betaine work together to improve GC-rich PCR amplification?
DMSO and betaine function through complementary biochemical mechanisms to facilitate the amplification of GC-rich templates:
DMSO (Dimethyl Sulfoxide) acts as a polar solvent that disrupts the secondary structures formed by GC-rich DNA sequences, particularly hairpin loops and stem-loop structures, by interfering with hydrogen bonding and base stacking interactions. This helps maintain DNA in a single-stranded state, making it more accessible to primers and polymerase [11] [69].
Betaine (also known as N,N,N-trimethylglycine) is an isostabilizing agent that equilibrates the differential melting temperatures between AT and GC base pairs. It reduces the kinetic barriers to DNA denaturation by eliminating the composition-dependent melting temperature variations across the template, thereby promoting more uniform strand separation during the denaturation steps [11] [69].
When used in combination, these additives work synergistically—DMSO directly destabilizes secondary structures while betaine reduces the overall energy required to denature double-stranded DNA, resulting in significantly improved amplification efficiency for GC-rich targets that are otherwise refractory to conventional PCR [4] [70].
What is the recommended starting protocol for implementing DMSO and betaine in PCR?
The following table provides a standardized starting protocol for implementing DMSO and betaine in a 50 μL PCR reaction mixture:
| Component | Final Concentration | Volume for 50 μL Reaction | Notes |
|---|---|---|---|
| 10X PCR Buffer | 1X | 5 μL | Standard buffer supplied with polymerase |
| dNTP Mix | 200 μM each | 1 μL of 10 mM stock | |
| MgClâ‚‚ | 1.5-4.0 mM | Variable | Optimize concentration based on template |
| Forward Primer | 0.1-1.0 μM | 1 μL of 20 μM stock | |
| Reverse Primer | 0.1-1.0 μM | 1 μL of 20 μM stock | |
| Template DNA | 1-1000 ng | Variable | 10^4-10^7 molecules |
| DMSO | 3-10% | 1.5-5 μL | Start with 5% |
| Betaine | 0.5-2.5 M | Variable | Start with 1.0-1.3 M |
| DNA Polymerase | 0.5-2.5 units | 0.5-2.5 μL | Follow manufacturer recommendations |
| Sterile Water | - | To 50 μL |
Thermal Cycling Conditions:
- Initial Denaturation: 94-98°C for 3-5 minutes
- Amplification Cycles (25-40 cycles):
- Denaturation: 94-98°C for 15-30 seconds
- Annealing: Temperature 3-5°C below primer Tm for 30 seconds
- Extension: 68-72°C for 1 minute per kb
- Final Extension: 68-72°C for 5-10 minutes [4] [6]
What are the common troubleshooting issues when using DMSO and betaine combinations?
| Problem | Possible Causes | Solutions |
|---|---|---|
| No Amplification | Additive concentration too high | Reduce DMSO to 3-5% and betaine to 0.5-1.0 M [26] |
| Polymerase inhibition | Use a polymerase known to be compatible with additives [69] | |
| Suboptimal Mg²⺠concentration | Titrate Mg²⺠concentration (1.0-4.0 mM) [69] | |
| Non-specific Bands | Annealing temperature too low | Increase annealing temperature in 2-5°C increments [71] [26] |
| Additive concentration too low | Increase betaine to 1.5-2.0 M to enhance specificity [4] | |
| Excessive cycle number | Reduce to 25-35 cycles [26] | |
| Reduced Yield | Polymerase activity affected | Increase polymerase amount by 25-50% [26] |
| Denaturation efficiency insufficient | Increase denaturation temperature or duration [26] | |
| Primer design issues | Verify primers lack secondary structures and have appropriate Tm [6] |
Can DMSO and betaine be used with other PCR enhancers?
Yes, DMSO and betaine can be effectively combined with other enhancers for particularly challenging templates:
7-deaza-dGTP: A dGTP analog that can be incorporated at up to 50 μM in combination with DMSO and betaine to further reduce secondary structure stability in GC-rich regions [4]. Note that 7-deaza-dGTP does not stain well with ethidium bromide, requiring alternative visualization methods [69].
Formamide: Can be used at 1.25-10% final concentration to increase primer annealing stringency, particularly when non-specific amplification persists despite DMSO and betaine optimization [6].
BSA (Bovine Serum Albumin): Addition of 10-100 μg/ml BSA can help counteract potential polymerase inhibition when using higher concentrations of additives, especially when amplifying from complex templates [6].
Research indicates that while ternary combinations can be effective, they should be approached systematically. One study found that while the combination of DMSO, betaine, and 7-deaza-dGTP was essential for amplifying particularly challenging GC-rich sequences (67-79% GC), combining DMSO and betaine together did not always provide additional benefit compared to DMSO alone in some systems [4] [15].
What is the evidence supporting the efficacy of DMSO and betaine combinations?
Multiple studies have quantitatively demonstrated the performance improvements achieved with DMSO and betaine:
| Study / Application | GC Content | Additives Used | Results |
|---|---|---|---|
| RET Promoter Amplification [4] | 79% (peaks to 90%) | 1.3 M Betaine + 5% DMSO + 50 μM 7-deaza-dGTP | Specific 392 bp product achieved; no product without additives |
| Plant ITS2 Barcoding [15] | Variable | 5% DMSO OR 1 M Betaine | PCR success rate increased from 42% to 100% across 50 species |
| LMX1B Gene Region [4] | 67.8% (peaks to 75.6%) | 1.3 M Betaine + 5% DMSO + 50 μM 7-deaza-dGTP | Clean specific product after non-specific amplification without additives |
| PHOX2B Exon 3 [4] | 72.7% | 1.3 M Betaine + 5% DMSO + 50 μM 7-deaza-dGTP | Enabled amplification of both alleles in heterozygous samples |
| De Novo Gene Synthesis [11] | High (IGF2R, BRAF) | DMSO and/or Betaine | Greatly improved target product specificity and yield during PCR amplification |
Figure 1: Decision workflow for troubleshooting DMSO and betaine in GC-rich PCR.
Research Reagent Solutions for DMSO and Betaine PCR Enhancement
| Reagent | Function | Recommended Starting Concentration |
|---|---|---|
| DMSO (Dimethyl Sulfoxide) | Disrupts secondary structures in GC-rich DNA | 5% (v/v) |
| Betaine | Equalizes melting temperatures of AT and GC base pairs | 1.0-1.3 M |
| 7-deaza-dGTP | dGTP analog that reduces secondary structure formation | 50 μM (partial substitution for dGTP) |
| MgClâ‚‚ | Essential cofactor for DNA polymerase activity | 1.5-2.0 mM (titrate as needed) |
| Hot-Start DNA Polymerase | Reduces non-specific amplification during reaction setup | 0.5-2.5 units/50 μL reaction |
| BSA (Bovine Serum Albumin) | Stabilizes polymerase and counteracts inhibitors | 10-100 μg/mL |
Figure 2: Mechanism of action for DMSO and betaine in GC-rich PCR.
Amplifying difficult DNA templates, particularly those with high GC-content, is a common and persistent challenge in molecular biology research. These sequences tend to form stable secondary structures and have high melting temperatures, which can lead to PCR failure, low yield, or non-specific amplification. While specialized polymerases and kits are available, they often represent a significant recurring cost. This guide outlines a highly effective, low-cost strategy utilizing a combination of two common chemical additives—Dimethyl Sulfoxide (DMSO) and Betaine—to overcome these challenges. This approach provides a high-impact, accessible solution that can enhance PCR success rates in most laboratories without the need for capital investment in new equipment or expensive proprietary enzyme mixes.
The Synergistic Mechanism of DMSO and Betaine The power of this method lies in the complementary actions of DMSO and Betaine, which together facilitate the amplification of GC-rich sequences that would otherwise resist efficient PCR.
- Betaine (also known as trimethylglycine) acts as an isostabilizer. It equilibrates the differential melting temperatures between AT-rich and GC-rich regions by neutralizing the base-pairing strength. This reduces the energy required to denature GC-rich stretches and helps to prevent the formation of secondary structures, such as hairpins, that can cause polymerase stalling [11] [72].
- DMSO aids in the denaturation of DNA by disrupting hydrogen bonding and base stacking interactions. It interferes with the formation of DNA secondary structures and can also lower the melting temperature (Tm) of the DNA duplex. It is reported that 10% DMSO can decrease the primer annealing temperature by approximately 5.5–6.0°C [73].
When used in combination, they exert a synergistic effect. A seminal study demonstrated that DMSO and betaine are "highly compatible" and "greatly improve de novo synthesis of GC-rich gene fragments," significantly improving both target product specificity and yield without requiring major protocol modifications [11].
Frequently Asked Questions (FAQs)
Q1: What is the typical working concentration for a DMSO and Betaine combination? For a standard PCR, a combination of 1-10% DMSO and 0.5 M to 2.5 M Betaine is effective [6] [72]. It is crucial to optimize the concentrations for your specific template and primer set. A good starting point is 5% DMSO and 1 M Betaine. The concentration of DMSO should be varied in 2% increments for optimization [73].
Q2: How do I incorporate these additives into my existing PCR protocol? Simply add the required volumes of DMSO and Betaine from stock solutions directly to your master mix. No other protocol modifications are strictly necessary, though adjusting the annealing temperature might be required. Remember that DMSO lowers the effective annealing temperature of the primers [73] [11].
Q3: Are DMSO and Betaine compatible with all DNA polymerases? Yes, both DMSO and Betaine are highly compatible with a wide range of DNA polymerases, including standard Taq and high-fidelity enzymes like Q5 [74] [11]. However, it is always prudent to consult the manufacturer's instructions for any specific warnings or recommendations.
Q4: What are the primary challenges in amplifying GC-rich sequences? GC-rich templates (≥60% GC content) are challenging due to the three hydrogen bonds in G-C base pairs, which make the DNA duplex more thermostable and resistant to denaturation. This promotes the formation of stable secondary structures (e.g., hairpins) that block polymerase progression and lead to poor yield or product failure [74].
Q5: Can I use a pre-made master mix that includes these enhancers? Yes, many manufacturers offer master mixes specifically tailored for GC-rich targets. These often contain proprietary blends of enhancers that may include compounds like DMSO and Betaine [74]. While convenient, these specialized mixes are typically more expensive than supplementing a standard polymerase with low-cost additives.
Troubleshooting Guide
This guide helps diagnose and resolve common issues encountered when setting up PCRs, particularly for difficult templates.
| Observation | Possible Cause | Recommended Solution |
|---|---|---|
| No Amplification or Low Yield | Poor template quality or degradation | Analyze DNA integrity by gel electrophoresis. Re-purify template if necessary to remove inhibitors like phenol or EDTA [26]. |
| Suboptimal annealing temperature | Recalculate primer Tm. Use a gradient cycler to optimize annealing temperature in 1-2°C increments. Increase temperature if non-specific, decrease if no product [26] [75]. | |
| Polymerase inhibition or insufficient amount | Further purify template or increase polymerase amount, especially if additives like DMSO are used [26]. | |
| Multiple or Non-Specific Bands | Annealing temperature too low | Increase annealing temperature stepwise to improve specificity [26] [76]. |
| Excess Mg2+ concentration | Optimize Mg2+ concentration in 0.2-1.0 mM increments. High Mg2+ promotes non-specific binding [26] [75]. | |
| Primer design issues or high concentration | Verify primer specificity and avoid complementarity at 3' ends. Optimize primer concentration (typically 0.1-1 μM) [26] [6]. | |
| Smear of Bands on Gel | Excessive cycle number | Reduce number of PCR cycles to prevent accumulation of non-specific amplicons [26]. |
| Contaminated reagents | Use fresh reagents, filter tips, and dedicate pre- and post-PCR work areas [27]. | |
| Incorrect Mg2+ concentration or poor template | Optimize Mg2+ and check template quality. Smearing can be caused by degraded DNA [27]. |
Quantitative Data & Protocols
Additive Concentration Table
The following table summarizes the standard concentrations for common PCR additives used to overcome various challenges.
| Additive | Typical Working Concentration | Primary Function | Template Specificity |
|---|---|---|---|
| DMSO | 3 - 10% [73] [6] | Disrupts secondary structures, reduces DNA Tm | GC-rich sequences [74] [11] |
| Betaine | 0.5 M - 2.5 M [6] [72] | Equalizes Tm of AT and GC base pairs, denaturant | GC-rich sequences [11] [72] |
| Formamide | 1.25 - 10% [6] | Increases primer annealing stringency | Improves specificity [74] |
| BSA | 10 - 100 μg/mL [6] | Binds to inhibitors, stabilizes polymerase | Inhibitor-rich samples (e.g., from blood) [27] |
Detailed Experimental Protocol: Combining DMSO and Betaine for GC-Rich PCR
This protocol is adapted from published research on the de novo synthesis of GC-rich genes and standard PCR enhancement methods [11] [6].
Research Reagent Solutions
| Item | Function in the Protocol |
|---|---|
| Template DNA | The target GC-rich DNA sequence to be amplified. |
| Specific Primers | Oligonucleotides designed to flank the target sequence. |
| High-Fidelity DNA Polymerase | Enzyme for accurate amplification, e.g., Q5 or similar. |
| 10X PCR Buffer | Provides optimal pH and salt conditions for the polymerase. |
| dNTP Mix | Building blocks (dATP, dCTP, dGTP, dTTP) for new DNA strands. |
| MgCl2 Solution | Essential cofactor for DNA polymerase activity. |
| Molecular Grade DMSO | Additive to disrupt DNA secondary structures. |
| Betaine (5M Stock) | Additive to homogenize DNA melting temperatures. |
| Nuclease-Free Water | Solvent to bring the reaction to the final volume. |
Step-by-Step Methodology
Prepare Master Mix: In a sterile, nuclease-free tube, combine the following reagents on ice. For multiple reactions, prepare a master mix to minimize pipetting error.
- Nuclease-Free Water: Q.S. to 50 μL final volume
- 10X PCR Buffer: 5 μL
- dNTP Mix (10 mM): 1 μL
- Forward Primer (20 μM): 1 μL
- Reverse Primer (20 μM): 1 μL
- MgCl2 (25 mM): Variable (e.g., 2-4 μL, see optimization note below)
- DMSO: 2.5 μL (for a 5% final concentration)
- Betaine (5M Stock): 10 μL (for a 1 M final concentration)
- DNA Polymerase: 0.5 - 2.5 units (per manufacturer's recommendation)
Add Template: Aliquot the master mix into individual PCR tubes. Then, add the template DNA (1-1000 ng, depending on complexity) to each tube. Mix the contents gently by pipetting up and down. Briefly centrifuge to collect the reaction at the bottom of the tube.
Thermal Cycling: Place the tubes in a thermal cycler and run the following optimized program:
- Initial Denaturation: 98°C for 30 seconds to 5 minutes (depending on polymerase and template complexity)
- 25-35 Cycles of:
- Denaturation: 98°C for 10-30 seconds
- Annealing: Temperature optimized for your primers (consider a 3-5°C reduction due to DMSO) for 15-30 seconds
- Extension: 72°C for 15-60 seconds/kb (depending on polymerase processivity)
- Final Extension: 72°C for 5-10 minutes
- Hold: 4°C forever
Analysis: Analyze the PCR product by agarose gel electrophoresis alongside an appropriate DNA molecular weight marker to verify amplicon size and yield.
Optimization Note: The Mg2+ concentration is a critical variable. While the supplied buffer may contain Mg2+, the presence of additives like DMSO and Betaine can affect its free concentration. It is recommended to perform a Mg2+ titration (e.g., testing 0.5 mM increments between 1.0 and 4.0 mM) to find the optimal concentration for your specific reaction [74].
Diagrams & Workflows
PCR Enhancement Mechanism
Experimental Workflow
Conclusion
The strategic, and often sequential, use of DMSO and betaine provides a powerful, low-cost, and highly accessible method to overcome the pervasive challenge of amplifying difficult DNA templates in PCR. Evidence from DNA barcoding and synthetic biology confirms that these additives can transform PCR success rates, making previously intractable targets routine. While they are not a universal panacea—and novel reagents like 'disruptors' are emerging for extreme cases—mastering DMSO and betaine should be a core competency in any molecular biology toolkit. For biomedical and clinical research, this translates to enhanced reliability in genotyping, pathogen detection, and the development of genetic therapies, ultimately accelerating discovery and diagnostic pipelines by ensuring data integrity from the most fundamental molecular level.
References
- 1. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOoq1Rsah9AcJ8_zRr49Kngu_8QJf5GY0b7Z3elt0UxiZ6XvqX8Op]
- 2. Having trouble when amplifying GC-rich sequences? [https://bionordika.no/science-hub/blog-articles/having-trouble-when-amplifying-gc-rich-sequences-here-are-four-tips-on-how-to-troubleshoot]
- 3. 5 easy tips to address problems amplifying GC-rich regions [https://bitesizebio.com/24002/problems-amplifying-gc-rich-regions-problem-solved/]
- 4. Betaine, Dimethyl Sulfoxide, and 7-Deaza-dGTP, a Powerful ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1876170/]
- 5. Optimization of PCR Conditions for Amplification of GC†... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6807403/]
- 6. Polymerase Chain Reaction: Basic Protocol Plus ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4846334/]
- 7. PCR Setup—Six Critical Components to Consider [https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-learning-center/invitrogen-school-of-molecular-biology/pcr-education/pcr-reagents-enzymes/pcr-component-considerations.html]
- 8. DMSO induces major morphological and physiological ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12360551/]
- 9. the molecular mechanism of action of dimethyl sulfoxide [https://pubmed.ncbi.nlm.nih.gov/17661513/]
- 10. Characterization of the structural and functional changes ... [https://www.sciencedirect.com/science/article/abs/pii/S0167483898000442]
- 11. DMSO and Betaine Greatly Improve Amplification of GC- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2883997/]
- 12. Betaine can eliminate the base pair composition dependence ... [https://pubmed.ncbi.nlm.nih.gov/8418834/]
- 13. DMSO and Betaine Greatly Improve Amplification of GC-Rich ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0011024]
- 14. The effects of DMSO on DNA conformations and mechanics [https://www.sciencedirect.com/science/article/pii/S0006349525004187]
- 15. DMSO and betaine significantly enhance the PCR ... [https://pubmed.ncbi.nlm.nih.gov/32433893/]
- 16. End-point PCR and PCR Primers Support - Troubleshooting [https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/pcr-cdna-synthesis-support-center/end-point-pcr-primers-support/end-point-pcr-primers-support-troubleshooting.html]
- 17. Disruptors, a new class of oligonucleotide reagents ... [https://www.sciencedirect.com/science/article/abs/pii/S0003269721000701]
- 18. Effects of DMSO, glycerol, betaine and their combinations ... [https://www.sciencedirect.com/science/article/abs/pii/S0731708516302473]
- 19. Is separating Pre-PCR and Post-PCR working areas ... [https://faqs.lexogen.com/faq/is-separating-pre-pcr-and-post-pcr-working-areas-n]
- 20. Could your PCR be affected by contamination? | IDT [https://www.idtdna.com/page/support-and-education/decoded-plus/could-your-pcr-be-affected-by-contamination/]
- 21. Can You Reuse PCR Tubes? Safety and Accuracy ... [https://synapse.patsnap.com/article/can-you-reuse-pcr-tubes-safety-and-accuracy-considerations]
- 22. Precautions of PCR Tubes In Experiments - Hawach [https://www.hawalab.com/news/precautions-of-pcr-tubes-in-experiments/]
- 23. Enhancement of DNA amplification efficiency by using ... [https://www.bocsci.com/blog/enhancement-of-dna-amplification-efficiency-by-using-pcr-additives/?srsltid=AfmBOorH2ec3Cop7ytL8NN_xd2z51CjVVB0-OA7VnKyM0m1u4_P2jrXr]
- 24. Sweet enhancers of polymerase chain reaction - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11521273/]
- 25. PCR Cycling Parameters—Six Key Considerations for ... [https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-learning-center/invitrogen-school-of-molecular-biology/pcr-education/pcr-reagents-enzymes/pcr-cycling-considerations.html]
- 26. PCR Troubleshooting Guide | Thermo Fisher Scientific - US [https://www.thermofisher.com/us/en/home/life-science/cloning/cloning-learning-center/invitrogen-school-of-molecular-biology/pcr-education/pcr-reagents-enzymes/pcr-troubleshooting.html]
- 27. PCR Troubleshooting: Common Problems and Solutions [https://www.mybiosource.com/learn/pcr-troubleshooting-common-problems-and-solutions/]
- 28. Optimizing PCR amplification of GC-rich nicotinic ... [https://www.sciencedirect.com/science/article/pii/S2405580825001396]
- 29. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOooBl6THuPavNTUiNEJYTCVg0ZcsM1Fz1UvM_L6zOQiB4yHOajSz]
- 30. Enhancement of DNA amplification efficiency by using ... [https://www.bocsci.com/blog/enhancement-of-dna-amplification-efficiency-by-using-pcr-additives/?srsltid=AfmBOorWlqbgaqh7awB-l_iu42uvOGQSDE_mmQcfBBMH84Ocyprbizkz]
- 31. Effects of DMSO , glycerol, betaine and their combinations in... [https://pubmed.ncbi.nlm.nih.gov/27284695/]
- 32. PCR Pitfalls: The Devil is in the Details [https://bitesizebio.com/35976/pcr-pitfalls-details/]
- 33. Troubleshooting your PCR [https://www.takarabio.com/learning-centers/pcr/faq/troubleshooting?srsltid=AfmBOorOk452MStJj1qwm8Ikk12SrBaG6EEjJGvp0JXdvCfhlhsVzfUB]
- 34. PCR Troubleshooting Guide [https://www.neb.com/en-us/tools-and-resources/troubleshooting-guides/pcr-troubleshooting-guide?srsltid=AfmBOoqd-lTW-2RQFGUFGaZfcsArzDYFe5RYXMNix59_u97I-yZsqkvS]
- 35. Betaine and DMSO : Enhancing Agents for PCR [https://www.semanticscholar.org/paper/Betaine-and-DMSO-%3A-Enhancing-Agents-for-PCR-Frackman-Kobs/0bc6fc3bcc9a6333b098fa1cc374e6388fc67ac1]
- 36. PCR Troubleshooting Guide [https://www.neb.com/en-us/tools-and-resources/troubleshooting-guides/pcr-troubleshooting-guide?srsltid=AfmBOorS_NDMWxeXIUFoOl0tn4c9AonbjcXaFzQTqYwHbengo_ouTbnD]
- 37. DNA Barcoding Troubleshooting: Fix PCR, Low Reads & ... [https://www.cd-genomics.com/pop-genomics/resources/dna-barcoding-troubleshooting.html]
- 38. DMSO and betaine greatly improve amplification of GC-rich ... [https://pubmed.ncbi.nlm.nih.gov/20552011/]
- 39. Optimizing your PCR [https://www.takarabio.com/learning-centers/pcr/faq/optimization?srsltid=AfmBOopxeztORCSjZYM2OSCYyy8hNXMpHnvgKLKoeBJflqBF20F2tiR1]
- 40. AccuPrimeâ„¢ GC-Rich DNA Polymerase, 200 rxns - FAQs [https://www.thermofisher.com/order/catalog/product/12337016/faqs]
- 41. Improved PCR Amplification of Broad Spectrum GC DNA ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0156478]
- 42. Enhancement of DNA amplification efficiency by using ... [https://www.bocsci.com/blog/enhancement-of-dna-amplification-efficiency-by-using-pcr-additives/?srsltid=AfmBOoqr8t6LEeHIEuXYt5FFvMgAEK7KBbsNG4c-pYt96UdVFBACNyRO]
- 43. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOorG3ZzQqkEn51ROT31uwiJdz9jVY_wYjxma3P0R8Ew6xtLI5dG6]
- 44. The enhancement of PCR amplification of a random ... [https://www.sciencedirect.com/science/article/abs/pii/S0165022X05000825]
- 45. PCR with Betaine, DMSO, BSA, DTT - Molecular Biology [http://www.protocol-online.org/biology-forums/posts/36331.html]
- 46. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOorM96MewotzMV8ymEnwfnDPMhRtLoh9yfgcO40GFkOf_lfWlvph]
- 47. Enhancement of DNA amplification efficiency by using ... [https://www.bocsci.com/blog/enhancement-of-dna-amplification-efficiency-by-using-pcr-additives/?srsltid=AfmBOopJsHVV_GL4xgmJGwlit_ttV1rQaeKF_j5GMlQcbv0OQgzaiiWN]
- 48. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOoqRQwo1kOfO-DSFAALB0GOPt87uRiWTHCMvtOiyZz4z8n-OhKKb]
- 49. Optimizing PCR: One Scientist's Not So Fond Memories [https://www.promegaconnections.com/optimizing-pcr-one-scientists-not-so-fond-memories/]
- 50. Amplification of GC-rich Putative Mouse PeP Promoter ... [https://www.ajmb.org/Article?id=101]
- 51. How to optimize PCR conditions for GC-rich regions? [https://synapse.patsnap.com/article/how-to-optimize-pcr-conditions-for-gc-rich-regions]
- 52. Why do I get smeared PCR products? [https://www.qiagen.com/us/resources/faq/87]
- 53. Weak PCR Band Results or Smearing - What To Do [https://www.goldbio.com/blogs/articles/troubleshooting-pcr-part-three-solutions-for-weak-bands-and-smearing?srsltid=AfmBOoqdl8bVP56YRfW27NHf2v0nGy3oay71cnQ4JpRAqEgDuyjfiLx0]
- 54. Optimizing PCR amplification of GC-rich nicotinic ... [https://pubmed.ncbi.nlm.nih.gov/40486497/]
- 55. DMSO increases mutation-scanning detection sensitivity in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5156568/]
- 56. Optimizing PCR: Proven Tips and Troubleshooting Tricks [https://www.the-scientist.com/optimizing-pcr-proven-tips-and-troubleshooting-tricks-71660]
- 57. Inhibit inhibition: PCR inhibition and how to prevent it [https://www.bioecho.com/blog/inhibit-inhibition-pcr-inhibition-and-how-to-prevent-it]
- 58. Optimizing PCR Reaction Conditions for High Fidelity and ... [https://www.labx.com/resources/optimizing-pcr-reaction-conditions-for-high-fidelity-and-yield/5579]
- 59. Sweet enhancers of polymerase chain reaction | PLOS One [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0311939]
- 60. Betaine, Dimethyl Sulfoxide, and 7-Deaza-dGTP, a ... [https://www.sciencedirect.com/science/article/abs/pii/S1525157810603431]
- 61. PCR Troubleshooting Guide [https://www.neb.com/en-us/tools-and-resources/troubleshooting-guides/pcr-troubleshooting-guide?srsltid=AfmBOor4jodw5KSEWO4EseGnQUiUpSVi2OCyc8wZETlB1_rBN5QNNL3n]
- 62. A comparison of four methods for PCR inhibitor removal [https://pubmed.ncbi.nlm.nih.gov/25553520/]
- 63. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOorcyHpDeTogHOpDXKkLaW1TBsvCrQQBAmFzOpksNqaew9qS16Hy]
- 64. A fundamental study of the PCR amplification of GC-rich ... [https://www.sciencedirect.com/science/article/abs/pii/S1476927108000881]
- 65. Troubleshooting your PCR [https://www.takarabio.com/learning-centers/pcr/faq/troubleshooting?srsltid=AfmBOoqV6Ou_SbaK8qv4akJ7uKa2fqPXi7DvrTYi2ADmjbTHuzkBs_X3]
- 66. PCR Troubleshooting Guide [https://www.neb.com/en-us/tools-and-resources/troubleshooting-guides/pcr-troubleshooting-guide?srsltid=AfmBOoqTqlHHV9LlKTAlGY6OPMSCarNLFsNBF_eKZx8B5SflC933Aip3]
- 67. Enhancement of DNA amplification efficiency by using ... [https://www.bocsci.com/blog/enhancement-of-dna-amplification-efficiency-by-using-pcr-additives/?srsltid=AfmBOoq4ZT2Q_5HqIXdtqc2nuSMBoPPhBYpNia2rJxruDwO5bn3SUnk0]
- 68. Article DMSO and betaine significantly enhance the PCR ... [https://www.sciencedirect.com/org/science/article/abs/pii/S0831279620000150]
- 69. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOoq6vz37NW15-sDImoc1tpiqj6Il-b404s2oKCZgvDC4X-m4aiva]
- 70. An efficient and economic enhancer mix for PCR [https://www.sciencedirect.com/science/article/abs/pii/S0006291X06014707]
- 71. End-point PCR and PCR Primers Support - Troubleshooting [https://www.thermofisher.com/kr/ko/home/technical-resources/technical-reference-library/pcr-cdna-synthesis-support-center/end-point-pcr-primers-support/end-point-pcr-primers-support-troubleshooting.html]
- 72. Types, mechanisms, and applications in long-range PCR [https://www.sciencedirect.com/science/article/abs/pii/S0300908422000499]
- 73. How much DMSO do I add to PCR? [https://www.aatbio.com/resources/faq-frequently-asked-questions/how-much-dmso-do-i-add-to-pcr]
- 74. Four tips for PCR amplification of GC-rich sequences [https://www.neb.com/en-us/nebinspired-blog/four-tips-for-pcr-amplification-of-gc-rich-sequences?srsltid=AfmBOopD41mDGmcRl_mdkHjZcs7AbhXLeJoVQfYlOQ2T6V81nmolICqW]
- 75. PCR Troubleshooting Guide [https://www.neb.com/en-us/tools-and-resources/troubleshooting-guides/pcr-troubleshooting-guide?srsltid=AfmBOoqlVMx2RbN9_2KxxiT2LiarRwdwCgAXfUlR0fUf9MZeBUtAqKmn]
- 76. PCR Troubleshooting Guide [https://www.genscript.com/pcr-troubleshooting-guide.html]